Regional Variations in Alirocumab Dosing Patterns in Patients with Heterozygous Familial Hypercholesterolemia During an Open-Label Extension Study
- PMID: 32363493
- PMCID: PMC7334259
- DOI: 10.1007/s10557-020-06984-0
Regional Variations in Alirocumab Dosing Patterns in Patients with Heterozygous Familial Hypercholesterolemia During an Open-Label Extension Study
Abstract
Purpose: During the alirocumab open-label extension study ODYSSEY OLE (open-label extension; NCT01954394), physicians could adjust alirocumab dosing for enrolled patients, who were diagnosed with heterozygous familial hypercholesterolemia (HeFH) and who had completed previous phase III clinical trials with alirocumab. This post hoc analysis evaluated the differences in physician-patient dosing decisions between the regions of Western Europe, Eastern Europe, North America, and the rest of the world (ROW).
Methods: Patients (n = 909) who received starting dose alirocumab 75 mg every 2 weeks (Q2W) during ODYSSEY OLE (patients from FH I, FH II, and LONG TERM parent studies) were included. Low-density lipoprotein cholesterol (LDL-C) levels were blinded until week 8; subsequently, LDL-C values were communicated to physicians. From week 12, dose adjustment from 75 to 150 mg Q2W, or vice versa, was possible.
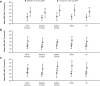
Results: Mean LDL-C values used for the decision to increase dose from 75 to 150 mg Q2W were higher in Eastern Europe (3.7 mmol/L; 144.0 mg/dL) and ROW (3.8 mmol/L; 145.2 mg/dL) compared with Western Europe (3.1 mmol/L; 118.6 mg/dL) and North America (3.3 mmol/L; 126.6 mg/dL). Irrespective of region, the mean LDL-C at the time of decision to maintain at 75 mg Q2W was approximately 1.8 mmol/L (70 mg/dL). During ODYSSEY OLE (median treatment duration of 131.7 weeks), alirocumab was shown to have no unexpected long-term safety concerns.
Conclusions: In this OLE study, the observed variations in clinical treatment decisions suggest that physicians may perceive the severity of HeFH and/or the treatment of HeFH differently depending on their region.
Keywords: Alirocumab; Dose adjustment; Familial hypercholesterolemia; LDL-C; Open-label extension; PCSK9.
Conflict of interest statement
Dr. Langslet has received speaker and expert witness fees from Sanofi, Amgen, and Boehringer Ingelheim. Professor Hovingh is a holder of a Vidi grant (016.156.445) from the Netherlands Organisation for Scientific Research (NWO) and from Klinkerpad; has received research support from Aegerion, Amgen, and Sanofi; has served as a consultant and received speaker fees from Amgen; Aegerion; Sanofi; Regeneron Pharmaceuticals, Inc.; and Pfizer; and has been employed part-time by Novo Nordisk AS, Copenhagen, Denmark, since April 2019. Professor Guyton has received research support from Sanofi, Regeneron Pharmaceuticals, Inc., Amgen, and Amarin; and has served as a consultant for Amgen, and FH Foundation. Drs. Baccara-Dinet and Letierce are stockholders and employees of Sanofi. Dr. Manvelian is a stockholder and an employee of Regeneron Pharmaceuticals, Inc. Dr. Farnier has received research support from Sanofi/Regeneron Pharmaceuticals, Inc.; Amgen; and Merck and Co; has served as a consultant for Sanofi/Regeneron Pharmaceuticals, Inc.; Pfizer; Amgen; Merck and Co; Eli Lilly; AstraZeneca; Kowa; Akcea/Ionis; Amarin; and Servier; and has received speaker fees from Sanofi/Regeneron Pharmaceuticals, Inc.; Abbott; Amgen; Merck and Co; Pfizer; and Mylan.
Figures

References
-
- Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–390a. doi: 10.1093/eurheartj/eht273. - DOI - PMC - PubMed
-
- Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, McCrindle B, Raal F, Rader D, Santos RD, Lopes-Virella M, Watts GF, Wierzbicki AS, American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167–2192. doi: 10.1161/CIR.0000000000000297. - DOI - PubMed
-
- Huijgen R, Kindt I, Verhoeven SB, Sijbrands EJ, Vissers MN, Kastelein JJ, et al. Two years after molecular diagnosis of familial hypercholesterolemia: majority on cholesterol-lowering treatment but a minority reaches treatment goal. PLoS One. 2010;5:e9220. doi: 10.1371/journal.pone.0009220. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

