Role of the superior ovarian nerve in the regulation of follicular development and steroidogenesis in the morning of diestrus 1
- PMID: 32363564
- PMCID: PMC7311564
- DOI: 10.1007/s10815-020-01787-6
Role of the superior ovarian nerve in the regulation of follicular development and steroidogenesis in the morning of diestrus 1
Abstract
Purpose: Little is known about the role of the superior ovarian nerve (SON) in follicular development during the estrus cycle. The aim of the present study was to analyze the role of neural signals arriving through the SON at the ovaries in the regulation of follicular development and ovarian steroid secretion in diestrus 1 of cyclic rats.
Methods: Cyclic rats were subjected to left, right, or bilateral SON sectioning or to unilateral or bilateral laparotomy at diestrus 1 at 11:00 h. Animals were sacrificed 24 h after surgery.
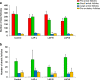
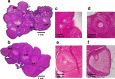
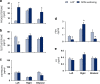
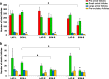
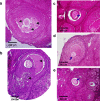
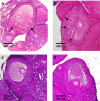
Results: Compared to laparotomized animals, unilateral SON sectioning decreased the number of preovulatory follicles, while bilateral SON sectioning resulted in a decreased number of atretic preantral follicles. An important observation was the presence of invaginations in the follicular wall of large antral and preovulatory follicles in animals with denervation. Furthermore, left SON sectioning increased progesterone levels but decreased testosterone levels, which are effects that were not observed in animals that were subjected to right denervation.
Conclusions: At 11:00 h of diestrus 1, the SON was found to stimulate follicle development, possibly via neural signals, such as noradrenaline and/or vasoactive intestinal peptide, and this stimulation induced the formation of follicle-stimulating hormone receptors. The role of the SON in the regulation of ovarian steroid secretion is asymmetric: the left SON inhibits the regulation of progesterone and stimulates testosterone secretion, and the right nerve does not participate in these processes.
Keywords: Follicular development; Gonadotropins; Noradrenaline; Ovarian steroidogenesis; Superior ovarian nerve.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

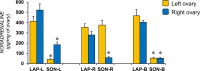
References
-
- Burden WH. The adrenergic innervation of mammalian ovaries. In: Ben-Jonathan N, Bahr JM, Weiner RI, editors. Catecholamines as hormone regulators. New York: Raven Press; 1985. pp. 261–278.
-
- Doganay M, Simsek A, Tapisiz OL, Mulazimoglu BS, Yumusak N, Gungor T. Superior ovarian nerve (SON) transaction leads to stunted follicular maturation: a histomorphologic and morphometric analysis in the rat model. Fertil Steril. 2010;93(5):1711–1714. - PubMed
-
- Dissen GA, Ojeda SR. Ovarian innervation. In: Knobil E, Neill JD, editors. Encyclopedia of reproduction. USA: Academic Press; 1999. pp. 583–589.
-
- Lawrence IE, Jr, Burden HW. The origin of the extrinsic adrenergic innervation to the rat ovary. Anat Rec. 1980;196:51–59. - PubMed
-
- Aguado LI, Ojeda SR. Prepuberal ovarian function is finely regulated by direct adrenergic influences. Role of noradrenergic innervation. Endocrinology. 1984;114(5):1845–1853. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

