Wireless recording from unrestrained monkeys reveals motor goal encoding beyond immediate reach in frontoparietal cortex
- PMID: 32364495
- PMCID: PMC7228770
- DOI: 10.7554/eLife.51322
Wireless recording from unrestrained monkeys reveals motor goal encoding beyond immediate reach in frontoparietal cortex
Erratum in
-
Correction: Wireless recording from unrestrained monkeys reveals motor goal encoding beyond immediate reach in frontoparietal cortex.Elife. 2021 Apr 13;10:e69225. doi: 10.7554/eLife.69225. Elife. 2021. PMID: 33847564 Free PMC article.
Abstract
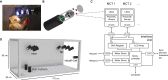
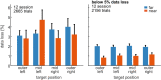
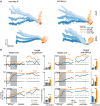
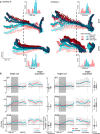
System neuroscience of motor cognition regarding the space beyond immediate reach mandates free, yet experimentally controlled movements. We present an experimental environment (Reach Cage) and a versatile visuo-haptic interaction system (MaCaQuE) for investigating goal-directed whole-body movements of unrestrained monkeys. Two rhesus monkeys conducted instructed walk-and-reach movements towards targets flexibly positioned in the cage. We tracked 3D multi-joint arm and head movements using markerless motion capture. Movements show small trial-to-trial variability despite being unrestrained. We wirelessly recorded 192 broad-band neural signals from three cortical sensorimotor areas simultaneously. Single unit activity is selective for different reach and walk-and-reach movements. Walk-and-reach targets could be decoded from premotor and parietal but not motor cortical activity during movement planning. The Reach Cage allows systems-level sensorimotor neuroscience studies with full-body movements in a configurable 3D spatial setting with unrestrained monkeys. We conclude that the primate frontoparietal network encodes reach goals beyond immediate reach during movement planning.
Keywords: arm movements; motion capture; motor cortex; neuroscience; parietal cortex; premotor cortex; rhesus macaque; wireless neurophysiology.
© 2020, Berger et al.
Conflict of interest statement
MB, NA, AG No competing interests declared
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

