PD-1 Blockade in Anaplastic Thyroid Carcinoma
- PMID: 32364844
- PMCID: PMC7476256
- DOI: 10.1200/JCO.19.02727
PD-1 Blockade in Anaplastic Thyroid Carcinoma
Abstract
Purpose: Anaplastic thyroid carcinoma is an aggressive malignancy that is almost always fatal and lacks effective systemic treatment options for patients with BRAF-wild type disease. As part of a phase I/II study in patients with advanced/metastatic solid tumors, patients with anaplastic thyroid carcinoma were treated with spartalizumab, a humanized monoclonal antibody against the programmed death-1 (PD-1) receptor.
Methods: We enrolled patients with locally advanced and/or metastatic anaplastic thyroid carcinoma in a phase II cohort of the study. Patients received 400 mg spartalizumab intravenously, once every 4 weeks. The overall response rate was determined according to RECIST v1.1.
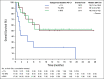
Results: Forty-two patients were enrolled. Adverse events were consistent with those previously observed with PD-1 blockade. Most common treatment-related adverse events were diarrhea (12%), pruritus (12%), fatigue (7%), and pyrexia (7%). The overall response rate was 19%, including three patients with a complete response and five with a partial response. Most patients had baseline tumor biopsies positive for PD-L1 expression (n = 28/40 evaluable), and response rates were higher in PD-L1-positive (8/28; 29%) versus PD-L1-negative (0/12; 0%) patients. The highest rate of response was observed in the subset of patients with PD-L1 ≥ 50% (6/17; 35%). Responses were seen in both BRAF-nonmutant and BRAF-mutant patients and were durable, with a 1-year survival of 52.1% in the PD-L1-positive population.
Conclusion: To our knowledge, this is the first clinical trial to show responsiveness of anaplastic thyroid carcinoma to PD-1 blockade.
Trial registration: ClinicalTrials.gov NCT02404441.
Figures


Comment in
-
Immunotherapy for Anaplastic Thyroid Carcinoma.J Clin Oncol. 2020 Aug 10;38(23):2603-2604. doi: 10.1200/JCO.20.01437. Epub 2020 Jun 18. J Clin Oncol. 2020. PMID: 32552275 No abstract available.
References
-
- Haddad RI, Nasr C, Bischoff L, et al. NCCN guidelines insights: Thyroid carcinoma, version 2.2018. J Natl Compr Canc Netw. 2018;16:1429–1440. - PubMed
-
- Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. - PubMed
-
- Tiedje V, Stuschke M, Weber F, et al. Anaplastic thyroid carcinoma: Review of treatment protocols. Endocr Relat Cancer. 2018;25:R153–R161. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

