A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose‑ranging study of the efficacy and safety of Astodrimer Gel for the treatment of bacterial vaginosis
- PMID: 32365097
- PMCID: PMC7197797
- DOI: 10.1371/journal.pone.0232394
A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose‑ranging study of the efficacy and safety of Astodrimer Gel for the treatment of bacterial vaginosis
Abstract
Background: Astodrimer Gel contains a novel dendrimer intended to treat and prevent bacterial vaginosis. We assessed the efficacy and safety of Astodrimer Gel for treatment of bacterial vaginosis.
Methods: 132 women with bacterial vaginosis were randomized 1:1:1:1 to Astodrimer 0.5% (N = 34), 1% (N = 33), or 3% (N = 32) Gel or hydroxyethyl cellulose placebo gel (N = 33) at a dose of 5 g vaginally once daily for 7 days at 6 centers in the United States. The primary endpoint was clinical cure (no bacterial vaginosis vaginal discharge and no more than one of 1) vaginal pH ≥4.5; 2) ≥20% clue cells; or 3) positive whiff test) at study days 21-30. Secondary analyses included clinical cure at study days 9-12, patient-reported symptoms, acceptability and adverse events.
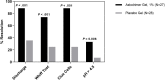
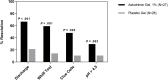
Results: The Astodrimer 1% Gel dose was superior to placebo for the primary and selected secondary efficacy measures in the modified intent-to-treat population. Clinical cure rates at day 9-12 were superior to placebo for the Astodrimer 3%, 1% and 0.5% Gel groups (62.5% [15/24; P = .002], 74.1% [20/27; P < .001], and 55.2% [16/29; P = .001], respectively, vs. 22.2% [6/27]). At day 21-30, clinical cure rates were 46.2% (12/26) for the 1% dose vs. 11.5% for placebo (3/26; P = .006). A greater proportion of patients reported absence of vaginal discharge and vaginal odor at day 9-12 and day 21-30 for Astodrimer Gel groups compared with placebo. Adverse events considered potentially treatment-related occurred in only 25% of Astodrimer Gel-treated patients vs. 22% of placebo patients.
Conclusion: Astodrimer Gel once daily for 7 days was superior to placebo for treatment of bacterial vaginosis and was well-tolerated. The 1% dose consistently showed the strongest efficacy across endpoints. These results support a role for Astodrimer Gel, 1%, as an effective treatment for bacterial vaginosis.
Conflict of interest statement
Starpharma is the developer of Astodrimer Gel, which has been licensed to and is now marketed by Aspen Pharmacare in Australia and New Zealand, and by Mundipharma in Europe and Asia. ASW received research funding from Gage Development Company. JRS is a paid consultant for Starpharma Pty Ltd, Talis One, Toltec, Lupin Pharmaceuticals, and Hologic. JRAP and SRE are paid employees of Starpharma Pty Ltd. CFP and AC were paid employees of Starpharma Pty Ltd and are now paid consultants for Starpharma Pty Ltd. PMcC and GRK are paid consultants for Starpharma Pty Ltd. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

