Treatment of cancer cells with Lapatinib negatively regulates general translation and induces stress granules formation
- PMID: 32365111
- PMCID: PMC7197775
- DOI: 10.1371/journal.pone.0231894
Treatment of cancer cells with Lapatinib negatively regulates general translation and induces stress granules formation
Abstract
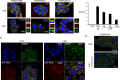
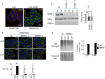
Stress granules (SG) are cytoplasmic RNA granules that form during various types of stress known to inhibit general translation, including oxidative stress, hypoxia, endoplasmic reticulum stress (ER), ionizing radiations or viral infection. Induction of these SG promotes cell survival in part through sequestration of proapoptotic molecules, resulting in the inactivation of cell death pathways. SG also form in cancer cells, but studies investigating their formation upon treatment with chemotherapeutics are very limited. Here we identified Lapatinib (Tykerb / Tyverb®), a tyrosine kinase inhibitor used for the treatment of breast cancers as a new inducer of SG in breast cancer cells. Lapatinib-induced SG formation correlates with the inhibition of general translation initiation which involves the phosphorylation of the translation initiation factor eIF2α through the kinase PERK. Disrupting PERK-SG formation by PERK depletion experiments sensitizes resistant breast cancer cells to Lapatinib. This study further supports the assumption that treatment with anticancer drugs activates the SG pathway, which may constitute an intrinsic stress response used by cancer cells to resist treatment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

