Induction of blood-circulating bile acids supports recovery from myelosuppressive chemotherapy
- PMID: 32365188
- PMCID: PMC7218440
- DOI: 10.1182/bloodadvances.2019000133
Induction of blood-circulating bile acids supports recovery from myelosuppressive chemotherapy
Abstract
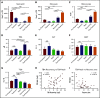
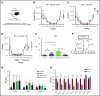
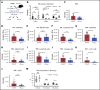
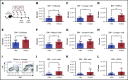
Chemotherapeutic agents can reduce bone marrow (BM) activity, causing myelosuppression, a common life-threatening complication of cancer treatment. It is challenging to predict the patients in whom prolonged myelosuppression will occur, resulting in a delay or discontinuation of the treatment protocol. An early indicator of recovery from myelosuppression would thus be highly beneficial in clinical settings. In this study, bile acids (BAs) were highly increased in the systemic circulation as a natural response during recovery from myelosuppression, supporting regeneration of BM cells. BA levels in the blood of pediatric cancer patients and mice treated with chemotherapeutic agents were increased, in synchrony with early proliferation of BM cells and recovery from myelosuppression. In a mouse model of altered BA composition, Cyp8b1 knockout mice, a subset of mice recovered poorly after chemotherapy. The poor recovery correlated with low levels and changes in composition of BAs in the liver and systemic circulation. Conversely, BA supplementation in chemotherapy-treated wild-type mice resulted in significantly improved recovery. The results suggest that part of the mechanism by which BAs support recovery is the suppression of endoplasmic reticulum stress pathways in expanding and recovering hematopoietic cells. The findings propose a novel role of BAs as early markers of recovery and active components of the recovery process after chemotherapy.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228-237. - PubMed
-
- van Os R, Robinson S, Sheridan T, Mauch PM. Granulocyte-colony stimulating factor impedes recovery from damage caused by cytotoxic agents through increased differentiation at the expense of self-renewal. Stem Cells. 2000;18(2):120-127. - PubMed
-
- Gardner RV. Long term hematopoietic damage after chemotherapy and cytokine. Front Biosci. 1999;4(5):e47-e57. - PubMed

