Selective Progesterone Receptor Modulators-Mechanisms and Therapeutic Utility
- PMID: 32365199
- PMCID: PMC8659360
- DOI: 10.1210/endrev/bnaa012
Selective Progesterone Receptor Modulators-Mechanisms and Therapeutic Utility
Abstract
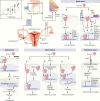
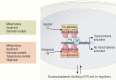
Selective progesterone receptor modulators (SPRMs) are a new class of compounds developed to target the progesterone receptor (PR) with a mix of agonist and antagonist properties. These compounds have been introduced for the treatment of several gynecological conditions based on the critical role of progesterone in reproduction and reproductive tissues. In patients with uterine fibroids, mifepristone and ulipristal acetate have consistently demonstrated efficacy, and vilaprisan is currently under investigation, while studies of asoprisnil and telapristone were halted for safety concerns. Mifepristone demonstrated utility for the management of endometriosis, while data are limited regarding the efficacy of asoprisnil, ulipristal acetate, telapristone, and vilaprisan for this condition. Currently, none of the SPRMs have shown therapeutic success in treating endometrial cancer. Multiple SPRMs have been assessed for efficacy in treating PR-positive recurrent breast cancer, with in vivo studies suggesting a benefit of mifepristone, and multiple in vitro models suggesting the efficacy of ulipristal acetate and telapristone. Mifepristone, ulipristal acetate, vilaprisan, and asoprisnil effectively treated heavy menstrual bleeding (HBM) in patients with uterine fibroids, but limited data exist regarding the efficacy of SPRMs for HMB outside this context. A notable class effect of SPRMs are benign, PR modulator-associated endometrial changes (PAECs) due to the actions of the compounds on the endometrium. Both mifepristone and ulipristal acetate are effective for emergency contraception, and mifepristone was approved by the US Food and Drug Administration (FDA) in 2012 for the treatment of Cushing's syndrome due to its additional antiglucocorticoid effect. Based on current evidence, SPRMs show considerable promise for treatment of several gynecologic conditions.
Keywords: asoprisnil; breast cancer; mifepristone; ulipristal acetate; uterine fibroid; vilaprisan.
© Endocrine Society 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





References
-
- Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18(4):502–519. - PubMed
-
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25(6):947–970. - PubMed
-
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8 (Suppl 1):3–63. - PubMed
-
- Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68(10–13):879–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

