Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY)
- PMID: 32365251
- PMCID: PMC7818402
- DOI: 10.1111/jdv.16558
Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY)
Abstract
Background: Secukinumab demonstrated superior efficacy over ustekinumab in the treatment of moderate to severe plaque psoriasis over 16 weeks in the CLARITY study and over 52 weeks in the CLEAR study.
Objective: To compare the efficacy and safety of secukinumab vs. ustekinumab over 52 weeks in CLARITY.
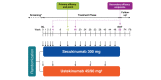
Methods: Analysis of 52-week data from CLARITY (NCT02826603), a phase 3b study in which patients were randomized to receive secukinumab 300 mg (n = 550) or ustekinumab 45/90 mg (n = 552) per label.
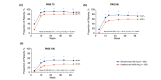
Results: At week 52, secukinumab was superior to ustekinumab in the proportion of patients who achieved ≥ 90% improvement in Psoriasis Area and Severity Index (73.2% vs. 59.8%; odds ratio [OR], 1.84 [95% CI, 1.41-2.41]; P < 0.0001), Investigator's Global Assessment modified 2011 responses of clear (0) or almost clear (1) skin (76.0% vs. 60.2%; OR, 2.12 [95% CI, 1.61-2.79]; P < 0.0001) and Dermatology Life Quality Index response of no effect (0/1) (69.9% vs. 61.2%; P = 0.0028). Proportions of patients with any adverse events were comparable between treatment arms.
Conclusions: This second head-to-head study confirmed the superior efficacy of secukinumab over ustekinumab in skin clearance and quality of life through 52 weeks, with safety comparable to that reported in previous trials. Clinicaltrials.gov identifier: NCT02826603.
© 2020 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures


References
-
- Ryan C, Korman NJ, Gelfand JM et al Research gaps in psoriasis: opportunities for future studies. J Am Acad Dermatol 2014; 70: 146–167. - PubMed
-
- Leonardi CL, Powers JL, Matheson RT et al Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003; 349: 2014–2022. - PubMed
-
- Menter A, Tyring SK, Gordon K et al Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58: 106–115. - PubMed
-
- Reich K, Nestle FO, Papp K et al Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005; 366: 1367–1374. - PubMed
-
- Saurat JH, Stingl G, Dubertret L et al Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008; 158: 558–566. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

