p66shc siRNA-Encapsulated PLGA Nanoparticles Ameliorate Neuropathic Pain Following Spinal Nerve Ligation
- PMID: 32365512
- PMCID: PMC7284875
- DOI: 10.3390/polym12051014
p66shc siRNA-Encapsulated PLGA Nanoparticles Ameliorate Neuropathic Pain Following Spinal Nerve Ligation
Abstract
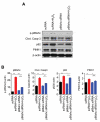
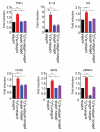
p66shc, a member of the shc adaptor protein family, has been shown to participate in regulation of mitochondrial homeostasis, apoptosis, and autophagosome formation. The present study was performed to investigate whether p66shc siRNA-encapsulated poly(d,l-lactic-co-glycolic acid) nanoparticles (p66shc siRNA-PLGA NPs) can attenuate spinal nerve ligation (SNL)-induced neuropathic pain in rats. The SNL-induced pain behavior was decreased in the p66shc siRNA-PLGA NP-treated group compared with the scrambled siRNA-PLGA NP-treated group. In the L5 spinal cord of the p66shc siRNA-PLGA NP-treated group, expression levels of phosphorylated p66shc, cleaved caspase-3, p62, and PINK1, as well as microglial activation, were also decreased. In addition, p66shc knockdown using p66shc siRNA reduced the expression levels of cleaved caspase-3, p62, and PINK1, as well as proinflammatory mediators in the H2O2-treated HT22 neuronal cells. These results suggest that downregulation of p66shc expression in the spinal cord using p66shc siRNA-PLGA NPs could reduce the SNL-induced neuropathic pain by attenuating the SNL-induced aberrant autophagic, mitophagic, and neuroinflammatory processes in rats.
Keywords: PLGA nanoparticle; autophagy; neuropathic pain; p66shc; proinflammatory mediators; spinal nerve ligation.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
CX3CR1-Targeted PLGA Nanoparticles Reduce Microglia Activation and Pain Behavior in Rats with Spinal Nerve Ligation.Int J Mol Sci. 2020 May 14;21(10):3469. doi: 10.3390/ijms21103469. Int J Mol Sci. 2020. PMID: 32423102 Free PMC article.
-
IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation.Int J Mol Sci. 2021 May 26;22(11):5657. doi: 10.3390/ijms22115657. Int J Mol Sci. 2021. PMID: 34073390 Free PMC article.
-
p38 siRNA-encapsulated PLGA nanoparticles alleviate neuropathic pain behavior in rats by inhibiting microglia activation.Nanomedicine (Lond). 2018 Jul;13(13):1607-1621. doi: 10.2217/nnm-2018-0054. Nanomedicine (Lond). 2018. PMID: 30028250
-
Intrathecal injection of phosphodiesterase 4B-specific siRNA attenuates neuropathic pain in rats with L5 spinal nerve ligation.Mol Med Rep. 2016 Feb;13(2):1914-22. doi: 10.3892/mmr.2015.4713. Epub 2015 Dec 23. Mol Med Rep. 2016. PMID: 26706904
-
Regulation of Selective B Cell Autophagy by the Pro-oxidant Adaptor p66SHC.Front Cell Dev Biol. 2020 Mar 26;8:193. doi: 10.3389/fcell.2020.00193. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32274384 Free PMC article. Review.
Cited by
-
RNA Interference Unleashed: Current Perspective of Small Interfering RNA (siRNA) Therapeutics in the Treatment of Neuropathic Pain.ACS Pharmacol Transl Sci. 2024 Sep 23;7(10):2951-2970. doi: 10.1021/acsptsci.4c00329. eCollection 2024 Oct 11. ACS Pharmacol Transl Sci. 2024. PMID: 39416962 Review.
-
Rejuvenating aged microglia by p16ink4a-siRNA-loaded nanoparticles increases amyloid-β clearance in animal models of Alzheimer's disease.Mol Neurodegener. 2024 Mar 16;19(1):25. doi: 10.1186/s13024-024-00715-x. Mol Neurodegener. 2024. PMID: 38493185 Free PMC article.
-
The Role of p66Shc in Cancer: Molecular Mechanisms and Therapeutic Implications.J Cell Mol Med. 2025 Jul;29(14):e70737. doi: 10.1111/jcmm.70737. J Cell Mol Med. 2025. PMID: 40690563 Free PMC article. Review.
-
Perampanel Reduces Brain Damage via Induction of M2 Microglia in a Neonatal Rat Stroke Model.Int J Nanomedicine. 2022 Jun 27;17:2791-2804. doi: 10.2147/IJN.S361377. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35782016 Free PMC article.
-
Mitochondrial Dysfunction in Oxidative Stress-Mediated Intervertebral Disc Degeneration.Orthop Surg. 2022 Aug;14(8):1569-1582. doi: 10.1111/os.13302. Epub 2022 Jun 8. Orthop Surg. 2022. PMID: 35673928 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

