Transient Conductive Hearing Loss Regulates Cross-Modal VGLUT Expression in the Cochlear Nucleus of C57BL/6 Mice
- PMID: 32365514
- PMCID: PMC7287693
- DOI: 10.3390/brainsci10050260
Transient Conductive Hearing Loss Regulates Cross-Modal VGLUT Expression in the Cochlear Nucleus of C57BL/6 Mice
Abstract
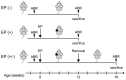
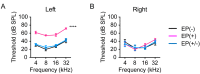
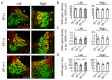
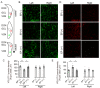
Auditory nerve fibers synapse onto the cochlear nucleus (CN) and are labeled using the vesicular glutamate transporter-1 (VGLUT-1), whereas non-auditory inputs are labeled using the VGLUT-2. However, the underlying regulatory mechanism of VGLUT expression in the CN remains unknown. We examined whether a sound level decrease, without primary neural damage, induces cellular and VGLUT expression change in the CN, and examined the potential for neural plasticity of the CN using unilateral conductive hearing loss models. We inserted earplugs in 8-week-old mice unilaterally for 4 weeks and subsequently removed them for another 4 weeks. Although the threshold of an auditory brainstem response significantly increased across all tested frequencies following earplug insertion, it completely recovered after earplug removal. Auditory deprivation had no significant impact on spiral ganglion and ventral CN (VCN) neurons' survival. Conversely, although the cell size and VGLUT-1 expression in the VCN significantly decreased after earplug insertion, VGLUT-2 expression in the granule cell lamina significantly increased. These cell sizes decreased and the alterations in VGLUT-1 and -2 expression almost completely recovered at 1 month after earplug removal. Our results suggested that the cell size and VGLUT expression in the CN have a neuroplasticity capacity, which is regulated by increases and decreases in sound levels. Restoration of the sound levels might partly prevent cell size decrease and maintain VGLUT expression in the CN.
Keywords: VGLUT; auditory deprivation; cochlear nucleus; neuroplasticity; synapse.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

