How Diffusion Impacts Cortical Protein Distribution in Yeasts
- PMID: 32365827
- PMCID: PMC7291136
- DOI: 10.3390/cells9051113
How Diffusion Impacts Cortical Protein Distribution in Yeasts
Abstract
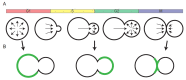
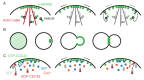
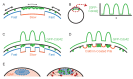
Proteins associated with the yeast plasma membrane often accumulate asymmetrically within the plane of the membrane. Asymmetric accumulation is thought to underlie diverse processes, including polarized growth, stress sensing, and aging. Here, we review our evolving understanding of how cells achieve asymmetric distributions of membrane proteins despite the anticipated dissipative effects of diffusion, and highlight recent findings suggesting that differential diffusion is exploited to create, rather than dissipate, asymmetry. We also highlight open questions about diffusion in yeast plasma membranes that remain unsolved.
Keywords: Cdc42; cell polarity; diffusion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

