Antimicrobial Isoflavones and Derivatives from Erythrina (Fabaceae): Structure Activity Perspective (Sar & Qsar) on Experimental and Mined Values Against Staphylococcus Aureus
- PMID: 32365905
- PMCID: PMC7277434
- DOI: 10.3390/antibiotics9050223
Antimicrobial Isoflavones and Derivatives from Erythrina (Fabaceae): Structure Activity Perspective (Sar & Qsar) on Experimental and Mined Values Against Staphylococcus Aureus
Abstract
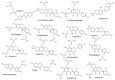
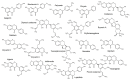
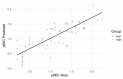
Prenylated (iso)flavonoids, -flavans and pterocarpans from taxa in Erythrina are repeatedly flagged as potent antimicrobial compounds. In the current study, bark from E. lysistemon was extracted and seven isoflavone derivatives were purified: erybraedin A (1), phaseollidin (2), abyssinone V-4' methyl ether (3), eryzerin C (4), alpumisoflavone (5), cristacarpin (6) and lysisteisoflavone (7). Minimum inhibition concentration (MIC) values were determined against a range of species of bacteria (skin pathogens), then values for another 67 derivatives from Erythrina, only against Staphylococcus aureus, were mined from the literature. Of the seven isolates, MIC values widely ranged from 1-600 μg/mL, with no obvious pattern of selectivity for Gram-types. Nevertheless, using the mined and experimentally determined values against S. aureus, Klekota-Roth fragments (Structure Activity Relationship: SAR) were determined then used as molecular descriptors to make a 'decision tree' based on structural characters inspired by the classes of antimicrobial potency (classes A-D). Furthermore, to make quantitative predictions of MIC values (Quantitative SAR: QSAR) 'pace regression' was utilized and validated (R² = 0.778, Q² = 0.727 and P² = 0.555). Evidently, the position and degree of prenylation is important; however, the presence of hydroxyl groups at positions 5 and 7 in ring A and 4' in ring B is associated with lower MIC values. While antimicrobial results continue to validate the traditional use of E. lysistemon extracts (or Erythrina generally) in therapeutic applications consistent with anti-infection, it is surprising that this class of compound is not being utilized more often in general industry applications, such as food or cosmetic preservation, or in topical antimicrobial creams. Prenylated (iso)flavonoids are derived from several other Genera, such as Dorstenia (Moraceae), Ficus (Moraceae), Glycyrrhiza (Fabaceae), Paulownia (Lamiales) or Pomifera (Moraceae).
Keywords: MRSA; QSAR; prenylated isoflavonoid; pterocarpan; traditional medicine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Lewis G., Schrire B., Mackinder B., Lock M. Legumes of the World. The Royal Botanic Gardens; Kew, UK: 2005.
-
- Telikepalli H., Gollapudi S.R., Keshavarz-Shokri A., Velazquez L., Sandmann R.A., Veliz E.A., Rao K.J., Madhavi A.S., Mitscher L.A. Isoflavonoids and a cinnamyl phenol from root extracts of Erythrina variegata. Phytochemistry. 1990;29:2005–2007. doi: 10.1016/0031-9422(90)85056-L. - DOI
-
- Mitscher L.A., Okwute S.K., Gollapudi S.R., Drake S., Avona E. Antimicrobial pterocarpans of Nigerian Erythrina mildbraedii. Phytochemistry. 1988;27:3449–3452. doi: 10.1016/0031-9422(88)80746-5. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

