Fecal microbiota composition is linked to the postoperative disease course in patients with Crohn's disease
- PMID: 32366222
- PMCID: PMC7197162
- DOI: 10.1186/s12876-020-01281-4
Fecal microbiota composition is linked to the postoperative disease course in patients with Crohn's disease
Abstract
Background: The role of the fecal microbiota composition for the postoperative disease course of patients with Crohn's disease (CD) who have undergone ileocecal resection remains to be established. In this study, we investigated if the fecal microbiota composition, determined by a high throughput test quantifying a pre-selected set of bacteria, is associated with the postoperative disease course of CD patients.
Methods: Fecal samples were obtained from healthy subjects as well as from CD patients, 3-10 weeks and 1 year after ileocaecal resection. The fecal microbial composition was analyzed by Genetic Analysis GA-map Dysbiosis test, targeting ≥300 bacteria on different taxonomic levels. Postoperative disease status was assessed endoscopically according to Rutgeerts scoring system 1 year after surgery. Differences in fecal microbiota composition between groups were analyzed by multivariate factor analyses and cluster analysis. Microbial stability over time was determined using Bray-Curtis dissimilarity.
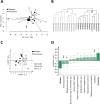
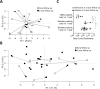
Results: One year after surgery, the fecal microbiota composition differed between CD patients (n = 21) and healthy subjects (n = 7). At this time point, the microbiota composition of CD patients was associated with disease course, clearly separating patients with disease relapse (n = 8) and patients in remission (n = 13). Further, the microbial within-patient stability was high during the first year after surgery, irrespective of disease course.
Conclusion: The fecal microbiota composition of CD patients, analyzed by GA-map Dysbiosis test, is subject to little variation over time, and may potentially be used as a non-invasive diagnostic tool for the postoperative disease course.
Keywords: Crohn’s disease; Fecal microbiota; Postoperative disease recurrence.
Conflict of interest statement
Lena Öhman is a member of the advisory board of Genetic Analysis. The remaining authors declare that they have no competing interest.
Figures


References
-
- Burr NE, Lord R, Hull MA, Subramanian V. Decreasing risk of first and subsequent surgeries in patients with Crohn’s disease in England from 1994 through 2013. Clin Gastroenterol Hepatol. 2019;17(10):2042–2049. - PubMed
-
- Rungoe C, Langholz E, Andersson M, Basit S, Nielsen NM, Wohlfahrt J, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut. 2014;63(10):1607–1616. - PubMed
-
- Domenech E, Garcia V, Iborra M, Gutierrez A, Garcia-Lopez S, Martin Arranz MD, et al. Incidence and Management of Recurrence in patients with Crohn's disease who have undergone intestinal resection: the Practicrohn study. Inflamm Bowel Dis. 2017;23(10):1840–1846. - PubMed
-
- Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn's disease recurrence. Aliment Pharmacol Ther. 2012;35(6):625–633. - PubMed
-
- Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105(2):289–297. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

