Malaria parasite plasmepsins: More than just plain old degradative pepsins
- PMID: 32366462
- PMCID: PMC7307202
- DOI: 10.1074/jbc.REV120.009309
Malaria parasite plasmepsins: More than just plain old degradative pepsins
Abstract
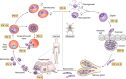
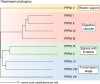
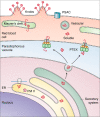
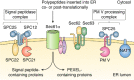
Plasmepsins are a group of diverse aspartic proteases in the malaria parasite Plasmodium Their functions are strikingly multifaceted, ranging from hemoglobin degradation to secretory organelle protein processing for egress, invasion, and effector export. Some, particularly the digestive vacuole plasmepsins, have been extensively characterized, whereas others, such as the transmission-stage plasmepsins, are minimally understood. Some (e.g. plasmepsin V) have exquisite cleavage sequence specificity; others are fairly promiscuous. Some have canonical pepsin-like aspartic protease features, whereas others have unusual attributes, including the nepenthesin loop of plasmepsin V and a histidine in place of a catalytic aspartate in plasmepsin III. We have learned much about the functioning of these enzymes, but more remains to be discovered about their cellular roles and even their mechanisms of action. Their importance in many key aspects of parasite biology makes them intriguing targets for antimalarial chemotherapy. Further consideration of their characteristics suggests that some are more viable drug targets than others. Indeed, inhibitors of invasion and egress offer hope for a desperately needed new drug to combat this nefarious organism.
Keywords: antimalarial chemotherapy; aspartic protease; digestive vacuole; hemoglobin; malaria; maturase; parasitology; plasmodium; protease; protozoan; transmission.
© 2020 Nasamu et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Francis S. E., Gluzman I. Y., Oksman A., Knickerbocker A., Mueller R., Bryant M. L., Sherman D. R., Russell D. G., and Goldberg D. E. (1994) Molecular characterization and inhibition of a Plasmodium falciparum aspartic hemoglobinase. EMBO J. 13, 306–317 10.1002/j.1460-2075.1994.tb06263.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

