HACE1 Prevents Lung Carcinogenesis via Inhibition of RAC-Family GTPases
- PMID: 32366477
- PMCID: PMC7611202
- DOI: 10.1158/0008-5472.CAN-19-2270
HACE1 Prevents Lung Carcinogenesis via Inhibition of RAC-Family GTPases
Abstract
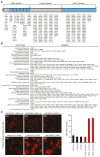
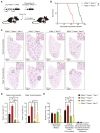
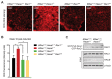
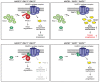
HACE1 is an E3 ubiquitin ligase with important roles in tumor biology and tissue homeostasis. Loss or mutation of HACE1 has been associated with the occurrence of a variety of neoplasms, but the underlying mechanisms have not been defined yet. Here, we report that HACE1 is frequently mutated in human lung cancer. In mice, loss of Hace1 led to enhanced progression of KRasG12D -driven lung tumors. Additional ablation of the oncogenic GTPase Rac1 partially reduced progression of Hace1-/- lung tumors. RAC2, a novel ubiquitylation target of HACE1, could compensate for the absence of its homolog RAC1 in Hace1-deficient, but not in HACE1-sufficient tumors. Accordingly, ablation of both Rac1 and Rac2 fully averted the increased progression of KRasG12D -driven lung tumors in Hace1-/- mice. In patients with lung cancer, increased expression of HACE1 correlated with reduced levels of RAC1 and RAC2 and prolonged survival, whereas elevated expression of RAC1 and RAC2 was associated with poor prognosis. This work defines HACE1 as a crucial regulator of the oncogenic activity of RAC-family GTPases in lung cancer development. SIGNIFICANCE: These findings reveal that mutation of the tumor suppressor HACE1 disrupts its role as a regulator of the oncogenic activity of RAC-family GTPases in human and murine lung cancer. GRAPHICAL ABSTRACT: http://cancerres.aacrjournals.org/content/canres/80/14/3009/F1.large.jpg.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. American Cancer Society. 2018;68:394–424. - PubMed
-
- Herbst RS, Morgensztern D, Boshoff C. Nature. Vol. 553. Nature Publishing Group; 2018. The biology and management of non-small cell lung cancer; pp. 446–54. - PubMed
-
- Walters S, Maringe C, Coleman MP, Peake MD, Butler J, Young N, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax. (7) 2013;68:551–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

