An Evidence Based Perspective on mRNA-SARS-CoV-2 Vaccine Development
- PMID: 32366816
- PMCID: PMC7218962
- DOI: 10.12659/MSM.924700
An Evidence Based Perspective on mRNA-SARS-CoV-2 Vaccine Development
Abstract
The first outbreak of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred in Wuhan, Hubei Province, China, in late 2019. The subsequent COVID-19 pandemic rapidly affected the health and economy of the world. The global approach to the pandemic was to isolate populations to reduce the spread of this deadly virus while vaccines began to be developed. In March 2020, the first phase I clinical trial of a novel lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine, mRNA-1273, which encodes the spike protein (S protein) of SARS-CoV-2, began in the United States (US). The production of mRNA-based vaccines is a promising recent development in the production of vaccines. However, there remain significant challenges in the development and testing of vaccines as rapidly as possible to control COVID-19, which requires international collaboration. This review aims to describe the background to the rationale for the development of mRNA-based SARS-CoV-2 vaccines and the current status of the mRNA-1273 vaccine.
Figures
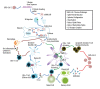
References
-
- World Health Organization (WHO) Novel Coronavirus (2019-nCoV) Situation Report-1. Jan 21, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- World Health Organization (WHO) Coronavirus (COVID-19) https://covid19.who.int.
-
- BASE Medicine Task Force. COVID-19: Facts and recommendations from A to Z. Sci Insigt. 2020;33(1):138–58.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

