Single cell and tissue-transcriptomic analysis of murine bladders reveals age- and TNFα-dependent but microbiota-independent tertiary lymphoid tissue formation
- PMID: 32366865
- PMCID: PMC7572484
- DOI: 10.1038/s41385-020-0290-x
Single cell and tissue-transcriptomic analysis of murine bladders reveals age- and TNFα-dependent but microbiota-independent tertiary lymphoid tissue formation
Abstract
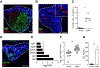
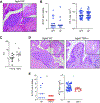
Aging has multifaceted effects on the immune system, but how aging affects tissue-specific immunity is not well-defined. Bladder diseases characterized by chronic inflammation are highly prevalent in older women, but mechanisms by which aging promotes these pathologies remain unknown. Tissue transcriptomics of unperturbed, young and aged bladders identified a highly altered immune landscape as a fundamental feature of the aging female bladder. Detailed mapping of immune cells using single cell RNA-sequencing revealed novel subsets of macrophages and dendritic cells and unique changes to the immune repertoire in the aged bladder. B and T cells are highly enriched in aged bladders and spontaneously form organized bladder tertiary lymphoid tissues (bTLTs). Naïve, activated, and germinal center B cells and IgA+ plasma cells are found within bTLT and associated with increased urinary IgA concentrations. bTLTs form with increasing age and their formation is dependent on TNFα. Microbiota are not required to form bTLT, as aged germfree mice also harbor them. Thus, bTLTs require age-dependent TNFα but are independent of the microbiota. Our results indicate that chronic, age-associated inflammation underlies a fundamental alteration to the bladder and establishes a resource for further investigation of the bladder immune system in homeostasis, aging, and disease.
Conflict of interest statement
Competing Interests
The authors have no financial interests to disclose.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

