Snakes elicit specific neural responses in the human infant brain
- PMID: 32366886
- PMCID: PMC7198620
- DOI: 10.1038/s41598-020-63619-y
Snakes elicit specific neural responses in the human infant brain
Abstract
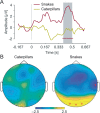
Detecting predators is essential for survival. Given that snakes are the first of primates' major predators, natural selection may have fostered efficient snake detection mechanisms to allow for optimal defensive behavior. Here, we provide electrophysiological evidence for a brain-anchored evolved predisposition to rapidly detect snakes in humans, which does not depend on previous exposure or knowledge about snakes. To do so, we recorded scalp electrical brain activity in 7- to 10-month-old infants watching sequences of flickering animal pictures. All animals were presented in their natural background. We showed that glancing at snakes generates specific neural responses in the infant brain, that are higher in amplitude than those generated by frogs or caterpillars, especially in the occipital region of the brain. The temporal dynamics of these neural responses support that infants devote increased attention to snakes than to non-snake stimuli. These results therefore demonstrate that a single fixation at snakes is sufficient to generate a prompt and large selective response in the infant brain. They argue for the existence in humans of an inborn, brain-anchored mechanism to swiftly detect snakes based on their characteristic visual features.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Isbell, L. A. The Fruit, the Tree, and the Serpent. (Harvard University Press, 2009).
-
- Öhman A, Mineka S. The malicious serpent: Snakes as a prototypical stimulus for an evolved module of fear. Curr. Dir. Psychol. Sci. 2003;12:5–9. doi: 10.1111/1467-8721.01211. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

