Homeostatic maintenance and age-related functional decline in the Drosophila ear
- PMID: 32366993
- PMCID: PMC7198581
- DOI: 10.1038/s41598-020-64498-z
Homeostatic maintenance and age-related functional decline in the Drosophila ear
Abstract
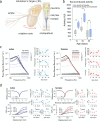
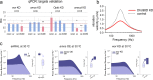
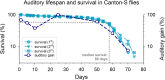
Age-related hearing loss (ARHL) is a threat to future human wellbeing. Multiple factors contributing to the terminal auditory decline have been identified; but a unified understanding of ARHL - or the homeostatic maintenance of hearing before its breakdown - is missing. We here present an in-depth analysis of homeostasis and ageing in the antennal ears of the fruit fly Drosophila melanogaster. We show that Drosophila, just like humans, display ARHL. By focusing on the phase of dynamic stability prior to the eventual hearing loss we discovered a set of evolutionarily conserved homeostasis genes. The transcription factors Onecut (closest human orthologues: ONECUT2, ONECUT3), Optix (SIX3, SIX6), Worniu (SNAI2) and Amos (ATOH1, ATOH7, ATOH8, NEUROD1) emerged as key regulators, acting upstream of core components of the fly's molecular machinery for auditory transduction and amplification. Adult-specific manipulation of homeostatic regulators in the fly's auditory neurons accelerated - or protected against - ARHL.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO. WHO Deafness and Hearing, http://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss (2018).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

