Advances in exploring activity cliffs
- PMID: 32367387
- PMCID: PMC7367915
- DOI: 10.1007/s10822-020-00315-z
Advances in exploring activity cliffs
Abstract
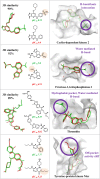
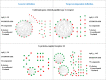
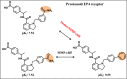
The activity cliff (AC) concept is of comparable relevance for medicinal chemistry and chemoinformatics. An AC is defined as a pair of structurally similar compounds with a large potency difference against a given target. In medicinal chemistry, ACs are of interest because they reveal small chemical changes with large potency effects, a concept referred to as structure-activity relationship (SAR) discontinuity. Computationally, ACs can be systematically identified, going far beyond individual compound series considered during lead optimization. Large-scale analysis of ACs has revealed characteristic features across many different compound activity classes. The way in which the molecular similarity and potency difference criteria have been addressed for defining ACs distinguishes between different generations of ACs and mirrors the evolution of the AC concept. We discuss different stages of this evolutionary path and highlight recent advances in AC research.
Keywords: Activity cliff concept; Activity data analysis; Cliff categories; Compound potency differences; Molecular similarity; Structure–activity relationships.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

