Prognostic value of HER2 status on circulating tumor cells in advanced-stage breast cancer patients with HER2-negative tumors
- PMID: 32367460
- PMCID: PMC7299127
- DOI: 10.1007/s10549-020-05662-x
Prognostic value of HER2 status on circulating tumor cells in advanced-stage breast cancer patients with HER2-negative tumors
Abstract
Purpose: Discordance between HER2 expression in tumor tissue (tHER2) and HER2 status on circulating tumor cells (cHER2) has been reported. It remains largely underexplored whether patients with tHER2-/cHER2+ can benefit from anti-HER2 targeted therapies.
Methods: cHER2 status was determined in 105 advanced-stage patients with tHER2- breast tumors. Association between cHER2 status and progression-free survival (PFS) was analyzed by univariate and multivariate Cox models and survival differences were compared by Kaplan-Meier method.
Results: Compared to the patients with low-risk cHER2 (cHER2+ < 2), those with high-risk cHER2 (cHER2+ ≥ 2) had shorter survival time and an increased risk for disease progression (hazard ratio [HR] 2.16, 95% confidence interval [CI] 1.20-3.88, P = 0.010). Among the patients with high-risk cHER2, those who received anti-HER2 targeted therapies had improved PFS compared with those who did not (HR 0.30, 95% CI 0.10-0.92, P = 0.035). In comparison, anti-HER2 targeted therapy did not affect PFS among those with low-risk cHER2 (HR 0.70, 95% CI 0.36-1.38, P = 0.306). Similar results were obtained after adjusting covariates. A longitudinal analysis of 67 patients with cHER2 detected during follow-ups found that those whose cHER2 status changed from high-risk at baseline to low-risk at first follow-up exhibited a significantly improved survival compared to those whose cHER2 remained high-risk (median PFS: 11.7 weeks vs. 2.0 weeks, log-rank P = 0.001).
Conclusion: In advanced-stage breast cancer patients with tHER2- tumors, cHER2 status has the potential to guide the use of anti-HER2 targeted therapy in patients with high-risk cHER2.
Keywords: Breast cancer; Circulating tumor cell (CTC); Human epidermal growth factor receptor 2 (HER2); Progression-free survival (PFS).
Conflict of interest statement
Figures

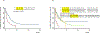

References
-
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. - PubMed
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN (2009) The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14:320–368. - PubMed
-
- Gingras I, Gebhart G, de Azambuja E, Piccart-Gebhart M (2017) HER2-positive breast cancer is lost in translation: time for patient-centered research. Nat Rev Clin Oncol 14:669–681. - PubMed
-
- Giordano SH, Temin S, Kirshner JJ, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, Gonzalez-Angulo AM, Krop I, Levinson J, Lin NU, Modi S, Patt DA, Perez EA, Perlmutter J, Ramakrishna N, Winer EP (2014) Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:2078–2099. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

