The lysosome: A potential juncture between SARS-CoV-2 infectivity and Niemann-Pick disease type C, with therapeutic implications
- PMID: 32367579
- PMCID: PMC7383733
- DOI: 10.1096/fj.202000654R
The lysosome: A potential juncture between SARS-CoV-2 infectivity and Niemann-Pick disease type C, with therapeutic implications
Abstract
Drug repurposing is potentially the fastest available option in the race to identify safe and efficacious drugs that can be used to prevent and/or treat COVID-19. By describing the life cycle of the newly emergent coronavirus, SARS-CoV-2, in light of emerging data on the therapeutic efficacy of various repurposed antimicrobials undergoing testing against the virus, we highlight in this review a possible mechanistic convergence between some of these tested compounds. Specifically, we propose that the lysosomotropic effects of hydroxychloroquine and several other drugs undergoing testing may be responsible for their demonstrated in vitro antiviral activities against COVID-19. Moreover, we propose that Niemann-Pick disease type C (NPC), a lysosomal storage disorder, may provide new insights into potential future therapeutic targets for SARS-CoV-2, by highlighting key established features of the disorder that together result in an "unfavorable" host cellular environment that may interfere with viral propagation. Our reasoning evolves from previous biochemical and cell biology findings related to NPC, coupled with the rapidly evolving data on COVID-19. Our overall aim is to suggest that pharmacological interventions targeting lysosomal function in general, and those particularly capable of reversibly inducing transient NPC-like cellular and biochemical phenotypes, constitute plausible mechanisms that could be used to therapeutically target COVID-19.
Keywords: COVID-19; angiotensin-converting enzyme-2 (ACE2); cathepsins; cholesterol; lipid rafts; lysosomal storage diseases; pandemic.
© 2020 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
All authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
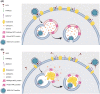
The reduced number and cholesterol‐depleted nature of lipid rafts in the plasma membrane of NPC cells influence the stability of ACE2 and TMPRSS2 which reside within these membrane domains.
The NPC‐related increase in plasma membrane levels of ADAM17 induces increased the shedding of ACE2, which hinders viral attachment/docking to host cells.
The NPC‐related abnormalities in the localization and activities of cathepsin L would blunt the chances of a successful viral fusion, after the endosome carrying the viral particle fuses with the NPC1‐deficient lysosome.
The elevated levels of the antiviral oxysterols 25‐HC and 7‐KC in NPC cells, also impede viral fusion and subsequent replication.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

