Artifacts and Anatomic Variations in Optical Coherence Tomography
- PMID: 32367701
- PMCID: PMC7204899
- DOI: 10.4274/tjo.galenos.2019.78000
Artifacts and Anatomic Variations in Optical Coherence Tomography
Abstract
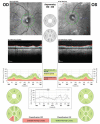
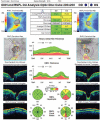
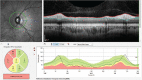
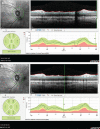
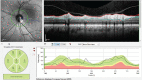
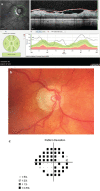
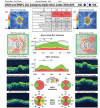
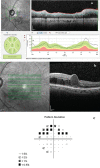
In recent years, ophthalmologists widely depend on optical coherence tomography (OCT), which is an objective, reliable, and repeatable structural test for both early diagnosis of glaucoma and detecting progression of the disease. Using this technology, it is now possible to take measures of various anatomic structures and layers of the optic nerve head, peripapillary retinal nerve fiber layer, and macular area. Although OCT has these powerful capabilities in general, anatomical variations, artifacts related to the ocular pathologies, and issues with image acquisition can be present in up to one-third of scans. These anatomical variations and artifacts can be misleading to an interpreter and may lead to erroneous conclusions. This review focuses on the realization and prevention of most common anatomical variations and artifacts observed with OCT imaging. The concepts of floor effect and red and green diseases are also investigated.
Keywords: OCT anatomic variations; OCT artifacts; Optical coherence tomography; green disease; red disease.
Conflict of interest statement
Figures




References
-
- Asrani S, Essaid L, Alder BD, Santiago-Turla C. Artifacts in spectraldomain optical coher-ence tomography measurements in glaucoma. JAMA Ophthalmol. 2014;132:396–402. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
