Synergistic and antagonistic drug interactions in the treatment of systemic fungal infections
- PMID: 32367801
- PMCID: PMC7200157
- DOI: 10.7554/eLife.54160
Synergistic and antagonistic drug interactions in the treatment of systemic fungal infections
Abstract
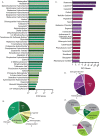
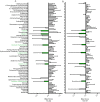
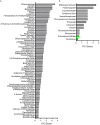
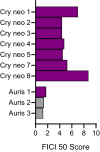
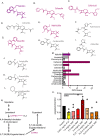
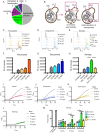
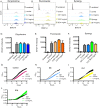
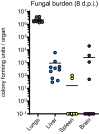
Invasive fungal infections cause 1.6 million deaths annually, primarily in immunocompromised individuals. Mortality rates are as high as 90% due to limited treatments. The azole class antifungal, fluconazole, is widely available and has multi-species activity but only inhibits growth instead of killing fungal cells, necessitating long treatments. To improve treatment, we used our novel high-throughput method, the overlap2 method (O2M) to identify drugs that interact with fluconazole, either increasing or decreasing efficacy. We identified 40 molecules that act synergistically (amplify activity) and 19 molecules that act antagonistically (decrease efficacy) when combined with fluconazole. We found that critical frontline beta-lactam antibiotics antagonize fluconazole activity. A promising fluconazole-synergizing anticholinergic drug, dicyclomine, increases fungal cell permeability and inhibits nutrient intake when combined with fluconazole. In vivo, this combination doubled the time-to-endpoint of mice with Cryptococcus neoformans meningitis. Thus, our ability to rapidly identify synergistic and antagonistic drug interactions can potentially alter the patient outcomes.
Keywords: Cryptococcus neoformans; drug combination; drug discovery; fungal infection; genetics; genomics; infectious disease; microbiology.
Plain language summary
Individuals with weakened immune systems – such as recipients of organ transplants – can fall prey to illnesses caused by fungi that are harmless to most people. These infections are difficult to manage because few treatments exist to fight fungi, and many have severe side effects. Antifungal drugs usually slow the growth of fungi cells rather than kill them, which means that patients must remain under treatment for a long time, or even for life. One way to boost efficiency and combat resistant infections is to combine antifungal treatments with drugs that work in complementary ways: the drugs strengthen each other’s actions, and together they can potentially kill the fungus rather than slow its progression. However, not all drug combinations are helpful. In fact, certain drugs may interact in ways that make treatment less effective. This is particularly concerning because people with weakened immune systems often take many types of medications. Here, Wambaugh et al. harnessed a new high-throughput system to screen how 2,000 drugs (many of which already approved to treat other conditions) affected the efficiency of a common antifungal called fluconazole. This highlighted 19 drugs that made fluconazole less effective, some being antibiotics routinely used to treat patients with weakened immune systems. On the other hand, 40 drugs boosted the efficiency of fluconazole, including dicyclomine, a compound currently used to treat inflammatory bowel syndrome. In fact, pairing dicyclomine and fluconazole more than doubled the survival rate of mice with severe fungal infections. The combined treatment could target many species of harmful fungi, even those that had become resistant to fluconazole alone. The results by Wambaugh et al. point towards better treatments for individuals with serious fungal infections. Drugs already in circulation for other conditions could be used to boost the efficiency of fluconazole, while antibiotics that do not decrease the efficiency of this medication should be selected to treat at-risk patients.
© 2020, Wambaugh et al.
Conflict of interest statement
MW, SD, MA, BB, MS, JB No competing interests declared
Figures






Similar articles
-
Small molecules restore azole activity against drug-tolerant and drug-resistant Candida isolates.mBio. 2023 Aug 31;14(4):e0047923. doi: 10.1128/mbio.00479-23. Epub 2023 Jun 16. mBio. 2023. PMID: 37326546 Free PMC article.
-
A repurposing approach identifies off-patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis.Eukaryot Cell. 2013 Feb;12(2):278-87. doi: 10.1128/EC.00314-12. Epub 2012 Dec 14. Eukaryot Cell. 2013. PMID: 23243064 Free PMC article.
-
Astemizole and an analogue promote fungicidal activity of fluconazole against Cryptococcus neoformans var. grubii and Cryptococcus gattii.Med Mycol. 2010 Mar;48(2):255-62. doi: 10.1080/13693780903081968. Med Mycol. 2010. PMID: 19572230
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Cryptococcus neoformans, a global threat to human health.Infect Dis Poverty. 2023 Mar 17;12(1):20. doi: 10.1186/s40249-023-01073-4. Infect Dis Poverty. 2023. PMID: 36932414 Free PMC article. Review.
Cited by
-
Searching for new antifungals for the treatment of cryptococcosis.Rev Soc Bras Med Trop. 2023 Jul 24;56:e01212023. doi: 10.1590/0037-8682-0121-2023. eCollection 2023. Rev Soc Bras Med Trop. 2023. PMID: 37493736 Free PMC article. Review.
-
Drug Target Elucidation Through Isolation and Analysis of Drug-Resistant Mutants in Cryptococcus neoformans.Methods Mol Biol. 2023;2658:127-143. doi: 10.1007/978-1-0716-3155-3_9. Methods Mol Biol. 2023. PMID: 37024699 Free PMC article.
-
Factoring in the Complexity of the Cystic Fibrosis Lung to Understand Aspergillus fumigatus and Pseudomonas aeruginosa Interactions.Pathogens. 2020 Aug 6;9(8):639. doi: 10.3390/pathogens9080639. Pathogens. 2020. PMID: 32781694 Free PMC article. Review.
-
Azole potentiation in Candida species.PLoS Pathog. 2023 Aug 31;19(8):e1011583. doi: 10.1371/journal.ppat.1011583. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37651385 Free PMC article.
-
Small molecules restore azole activity against drug-tolerant and drug-resistant Candida isolates.mBio. 2023 Aug 31;14(4):e0047923. doi: 10.1128/mbio.00479-23. Epub 2023 Jun 16. mBio. 2023. PMID: 37326546 Free PMC article.
References
-
- Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, Nieuwkoop AJ, Comellas G, Maryum N, Wang S, Uno BE, Wildeman EL, Gonen T, Rienstra CM, Burke MD. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nature Chemical Biology. 2014;10:400–406. doi: 10.1038/nchembio.1496. - DOI - PMC - PubMed
-
- Applen Clancey S, Ciccone EJ, Coelho MA, Davis J, Ding L, Betancourt R, Glaubiger S, Lee Y, Holland SM, Gilligan P, Sung J, Heitman J. Cryptococcus deuterogattii VGIIa infection associated with travel to the Pacific northwest outbreak region in an Anti-Granulocyte-Macrophage Colony-Stimulating factor Autoantibody-Positive patient in the united states. mBio. 2019;10:e02733. doi: 10.1128/mBio.02733-18. - DOI - PMC - PubMed
-
- Arthington-Skaggs BA, Lee-Yang W, Ciblak MA, Frade JP, Brandt ME, Hajjeh RA, Harrison LH, Sofair AN, Warnock DW, Candidemia Active Surveillance Group Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing candida isolates. Antimicrobial Agents and Chemotherapy. 2002;46:2477–2481. doi: 10.1128/AAC.46.8.2477-2481.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

