Nanoparticle Drug Delivery System for Glioma and Its Efficacy Improvement Strategies: A Comprehensive Review
- PMID: 32368041
- PMCID: PMC7173867
- DOI: 10.2147/IJN.S243223
Nanoparticle Drug Delivery System for Glioma and Its Efficacy Improvement Strategies: A Comprehensive Review
Abstract
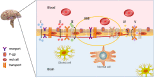
Gliomas are the most common tumor of the central nervous system. However, the presence of the brain barrier blocks the effective delivery of drugs and leads to the treatment failure of various drugs. The development of a nanoparticle drug delivery system (NDDS) can solve this problem. In this review, we summarized the brain barrier (including blood-brain barrier (BBB), blood-brain tumor barriers (BBTB), brain-cerebrospinal fluid barrier (BCB), and nose-to-brain barrier), NDDS of glioma (such as passive targeting systems, active targeting systems, and environmental responsive targeting systems), and NDDS efficacy improvement strategies and deficiencies. The research prospect of drug-targeted delivery systems for glioma is also discussed.
Keywords: brain barrier; deficiencies of NDDS; efficacy improvement strategies; nanoparticle drug delivery system; glioma.
© 2020 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- Norden AD, Drappatz J, Wen PY. Malignant Gliomas in Adults. Blue Books Neurol. 2010;36(10):99–120.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

