Antiviral Activity of Chitosan Nanoparticles Encapsulating Curcumin Against Hepatitis C Virus Genotype 4a in Human Hepatoma Cell Lines
- PMID: 32368050
- PMCID: PMC7184126
- DOI: 10.2147/IJN.S241702
Antiviral Activity of Chitosan Nanoparticles Encapsulating Curcumin Against Hepatitis C Virus Genotype 4a in Human Hepatoma Cell Lines
Erratum in
-
Erratum: Antiviral Activity of Chitosan Nanoparticles Encapsulating Curcumin Against Hepatitis C Virus Genotype 4a in Human Hepatoma Cell Lines [Corrigendum].Int J Nanomedicine. 2021 Mar 5;16:1927. doi: 10.2147/IJN.S308439. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33707947 Free PMC article.
Retraction in
-
Antiviral Activity of Chitosan Nanoparticles Encapsulating Curcumin Against Hepatitis C Virus Genotype 4a in Human Hepatoma Cell Lines [Retraction].Int J Nanomedicine. 2022 Jul 5;17:2891-2892. doi: 10.2147/IJN.S380656. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35818402 Free PMC article.
Abstract
Purpose: Current direct-acting antiviral agents for treatment of hepatitis C virus genotype 4a (HCV-4a) have been reported to cause adverse effects, and therefore less toxic antivirals are needed. This study investigated the role of curcumin chitosan (CuCs) nanocomposite as a potential anti-HCV-4a agent in human hepatoma cells Huh7.
Methods: Docking of curcumin and CuCs nanocomposite and binding energy calculations were carried out. Chitosan nanoparticles (CsNPs) and CuCs nanocomposite were prepared with an ionic gelation method and characterized with TEM, zeta size and potential, and HPLC to calculate encapsulation efficiency. Cytotoxicity studies were performed on Huh7 cells using MTT assay and confirmed with cellular and molecular assays. Anti-HCV-4a activity was determined using real-time PCR and Western blot.
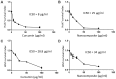
Results: The strength of binding interactions between protein ligand complexes gave scores with NS3 protease, NS5A polymerase, and NS5B polymerase of -124.91, -159.02, and -129.16, for curcumin respectively, and -68.51, -54.52, and -157.63 for CuCs nanocomposite, respectively. CuCs nanocomposite was prepared at sizes 29-39.5 nm and charges of 33 mV. HPLC detected 4% of curcumin encapsulated into CsNPs. IC50 was 8 µg/mL for curcumin and 25 µg/mL for the nanocomposite on Huh7 but was 25.8 µg/mL and 34 µg/mL on WISH cells. CsNPs had no cytotoxic effect on tested cell lines. Apoptotic genes' expression revealed the caspase-dependent pathway mechanism. CsNPs and CuCs nanocomposite demonstrated 100% inhibition of viral entry and replication, which was confirmed with HCV core protein expression.
Conclusion: CuCs nanocomposite inhibited HCV-4a entry and replication compared to curcumin alone, suggesting its potential role as an effective therapeutic agent.
Keywords: Huh7; caspase-dependent pathway; chitosan curcumin nanocomposite; docking; hepatitis C virus genotype 4a.
© 2020 Loutfy et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures








References
-
- World Health Organization. Hepatitis C; 2019. Available from: http://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed July22.
-
- Tremblay N, Young Park A, Lamarre D. HCV NS3/4A protease inhibitors and the road to effective direct-acting antiviral therapies. Hepatitis C Virus II. 2016. 257–285.
-
- Nafisi S, Roy S, Gish R, Manch R, Kohli A. Defining the possibilities: is short duration treatment of chronic hepatitis C genotype 1 with sofosbuvir-containing regimens likely to be as effective as current regimens? Expert Rev Anti Infect Ther. 2016;14(1):41–56. doi: 10.1586/14787210.2016.1114883 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

