Enhancing Drug Delivery for Overcoming Angiogenesis and Improving the Phototherapy Efficacy of Glioblastoma by ICG-Loaded Glycolipid-Like Micelles
- PMID: 32368051
- PMCID: PMC7184138
- DOI: 10.2147/IJN.S234240
Enhancing Drug Delivery for Overcoming Angiogenesis and Improving the Phototherapy Efficacy of Glioblastoma by ICG-Loaded Glycolipid-Like Micelles
Abstract
Background: Phototherapy is a potential new candidate for glioblastoma (GBM) treatment. However inadequate phototherapy due to stability of the photosensitizer and low target specificity induces the proliferation of neovascular endothelial cells for angiogenesis and causes poor prognosis.
Methods: In this study, we constructed c(RGDfk)-modified glycolipid-like micelles (cRGD-CSOSA) encapsulating indocyanine green (ICG) for dual-targeting neovascular endothelial cells and tumor cells, and cRGD-CSOSA/ICG mediated dual effect of PDT/PTT with NIR irradiation.
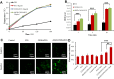
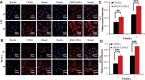
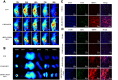
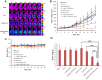
Results: In vitro, cRGD-CSOSA/ICG inhibited cell proliferation and blocked angiogenesis with NIR irradiation. In vivo, cRGD-CSOSA/ICG exhibited increased accumulation in neovascular endothelial cells and tumor cells. Compared with that of CSOSA, the accumulation of cRGD-CSOSA in tumor tissue was further improved after dual-targeted phototherapy pretreatment. With NIR irradiation, the tumor-inhibition rate of cRGD-CSOSA/ICG was 80.00%, significantly higher than that of ICG (9.08%) and CSOSA/ICG (42.42%). Histological evaluation showed that the tumor vessels were reduced and that the apoptosis of tumor cells increased in the cRGD-CSOSA/ICG group with NIR irradiation.
Conclusion: The cRGD-CSOSA/ICG nanoparticle-mediated dual-targeting phototherapy could enhance drug delivery to neovascular endothelial cells and tumor cells for anti-angiogenesis and improve the phototherapy effect of glioblastoma, providing a new strategy for glioblastoma treatment.
Keywords: angiogenesis; dual-targeting; glioblastoma; glycolipid-like micelles; phototherapy.
© 2020 Liu et al.
Conflict of interest statement
There are no conflicts of interest to declare in this work.
Figures





Similar articles
-
Mitochondrial alkaline pH-responsive drug release mediated by Celastrol loaded glycolipid-like micelles for cancer therapy.Biomaterials. 2018 Feb;154:169-181. doi: 10.1016/j.biomaterials.2017.07.036. Epub 2017 Jul 31. Biomaterials. 2018. PMID: 29128845
-
Glycyrrhetinic acid and RGD dual-targeted liposomes for combined chemotherapy and phototherapy in liver cancer.Int J Pharm. 2025 Aug 20;681:125779. doi: 10.1016/j.ijpharm.2025.125779. Epub 2025 Jun 1. Int J Pharm. 2025. PMID: 40456423
-
cRGD-directed, NIR-responsive and robust AuNR/PEG-PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo.J Control Release. 2014 Dec 10;195:63-71. doi: 10.1016/j.jconrel.2014.07.054. Epub 2014 Aug 7. J Control Release. 2014. PMID: 25108151
-
Enhancing Photothermal Therapy Efficacy by In Situ Self-Assembly in Glioma.ACS Appl Mater Interfaces. 2023 Jan 11;15(1):57-66. doi: 10.1021/acsami.2c14413. Epub 2022 Oct 7. ACS Appl Mater Interfaces. 2023. PMID: 36206382 Free PMC article. Review.
-
Photosensitizer-based small molecule theranostic agents for tumor-targeted monitoring and phototherapy.Bioorg Chem. 2023 Jul;136:106554. doi: 10.1016/j.bioorg.2023.106554. Epub 2023 Apr 19. Bioorg Chem. 2023. PMID: 37094481 Review.
Cited by
-
Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM).Cancers (Basel). 2023 Apr 1;15(7):2116. doi: 10.3390/cancers15072116. Cancers (Basel). 2023. PMID: 37046777 Free PMC article. Review.
-
Current Photodynamic Therapy for Glioma Treatment: An Update.Biomedicines. 2024 Feb 6;12(2):375. doi: 10.3390/biomedicines12020375. Biomedicines. 2024. PMID: 38397977 Free PMC article. Review.
-
Current Non-Metal Nanoparticle-Based Therapeutic Approaches for Glioblastoma Treatment.Biomedicines. 2024 Aug 11;12(8):1822. doi: 10.3390/biomedicines12081822. Biomedicines. 2024. PMID: 39200286 Free PMC article. Review.
-
A Novel Multi-Effect Photosensitizer for Tumor Destruction via Multimodal Imaging Guided Synergistic Cancer Phototherapy.Int J Nanomedicine. 2024 Jun 24;19:6377-6397. doi: 10.2147/IJN.S461843. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38952677 Free PMC article.
-
The role of cell membrane-coated nanoparticles as a novel treatment approach in glioblastoma.Front Mol Biosci. 2023 Jan 4;9:1083645. doi: 10.3389/fmolb.2022.1083645. eCollection 2022. Front Mol Biosci. 2023. PMID: 36660431 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

