Chrysin Induced Cell Apoptosis and Inhibited Invasion Through Regulation of TET1 Expression in Gastric Cancer Cells
- PMID: 32368086
- PMCID: PMC7182457
- DOI: 10.2147/OTT.S246031
Chrysin Induced Cell Apoptosis and Inhibited Invasion Through Regulation of TET1 Expression in Gastric Cancer Cells
Erratum in
-
Erratum: Chrysin Induced Cell Apoptosis and Inhibited Invasion Through Regulation of TET1 Expression in Gastric Cancer Cells [Corrigendum].Onco Targets Ther. 2021 Jan 26;14:697-698. doi: 10.2147/OTT.S302177. eCollection 2021. Onco Targets Ther. 2021. PMID: 33531817 Free PMC article.
Abstract
Objective: Ten-eleven translocation (TET) enzymes that oxidize a 5-methylcytosine (5mC) to yield 5-hydroxymethylcytosine (5hmC) have been responsible for fine-tuning methylation patterns and exhibit role in epigenetic modifications. Chrysin, a natural flavone frequently present in honey, has been recognized to exhibit anti-tumor properties. In this study, we investigated the effects of Chrysin in the expression pattern of TET proteins in gastric cancer (GC) cells.
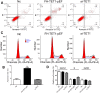
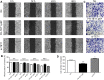
Materials and methods: Using qRT-PCR and Western blot analysis, we analyzed the expression of TET1 in GC cells in vitro following treatment with Chrysin. Immunofluorescence staining detected the expression levels of 5mC and 5hmC. Flow cytometry, wound healing, and Matrigel invasion assays were performed to determine cell proliferation, cell cycle, apoptosis, and migration and invasion of GC cells following treatment with Chrysin, si-TET1, and TET1-KO. Furthermore, a xenograft model was developed to analyze the expression pattern of TET1 on tumor development in vivo.
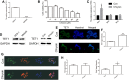
Results: qRT-PCR and Western blot assays indicated that treatment with Chrysin significantly promoted the expression of TET1 in GC cells. Immunofluorescence study further confirmed that TET1 and 5hmC levels were significantly enhanced following treatment with Chrysin in MKN45 cells. Moreover, our results suggested that Chrysin could noticeably induce cell apoptosis and inhibit cell migration and invasion. Further, knockdown and overexpression of TET1 were conducted to investigate whether TET1 expression affected cell apoptosis, and cell migration and invasion in MKN45 cells. The results indicated that overexpression of TET1 markedly promoted cell apoptosis and inhibited cell migration and invasion. Furthermore, the TET1 gene knocked out was generated using the CRISPR/Cas9 system. Our data suggested that TET1 expression was associated with GC tumor growth in vivo.
Conclusion: This study indicated that Chrysin exerted anti-tumor effects through the regulation of TET1 expression in GC and presented TET1 as a novel promising therapeutic target for GC therapy.
Keywords: 5hmC; Chrysin; TET1; cell apoptosis; cell invasion.
© 2020 Zhong et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

