Copper-catalysed benzylic C-H coupling with alcohols via radical relay enabled by redox buffering
- PMID: 32368720
- PMCID: PMC7197765
- DOI: 10.1038/s41929-020-0425-1
Copper-catalysed benzylic C-H coupling with alcohols via radical relay enabled by redox buffering
Abstract
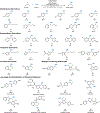
Cross-coupling reactions enable rapid, convergent synthesis of diverse molecules and provide the foundation for modern chemical synthesis. The most widely used methods employ sp2-hybridized coupling partners, such as aryl halides or related pre-functionalized substrates. Here, we demonstrate copper-catalysed oxidative cross coupling of benzylic C-H bonds with alcohols to afford benzyl ethers, enabled by a redox-buffering strategy that maintains the activity of the copper catalyst throughout the reaction. The reactions employ the C-H substrate as the limiting reagent and exhibit broad scope with respect to both coupling partners. This approach to direct site-selective functionalization of C(sp3)-H bonds provides the basis for efficient three-dimensional diversification of organic molecules and should find widespread utility in organic synthesis, particularly for medicinal chemistry applications.
Conflict of interest statement
Competing Interests. The authors declare no competing interests.
Figures





References
-
- Brown DG & Boström J Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone?: miniperspective. J. Med. Chem 59, 4443–4458 (2016). - PubMed
-
- Boström J, Brown DG, Young RJ & Keserü GM Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Disc. 17, 709–727 (2018). - PubMed
-
- Lovering F, Bikker J & Humblet C Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem 52, 6752–6756 (2009). - PubMed
-
- Blakemore DC et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem 10, 383–394 (2018). - PubMed
-
- Cernak T, Dykstra KD, Tyagarajan S, Vachal P & Krska SW The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev 45, 546–576 (2016). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
