Therapeutic efficacy of environmental enrichment on behavioral, endocrine, and synaptic alterations in an animal model of maternal immune activation
- PMID: 32368757
- PMCID: PMC7197879
- DOI: 10.1016/j.bbih.2020.100043
Therapeutic efficacy of environmental enrichment on behavioral, endocrine, and synaptic alterations in an animal model of maternal immune activation
Abstract
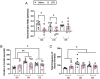
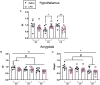
Maternal immune activation (MIA) has been identified as a significant risk factor for several neurodevelopmental disorders. We have previously demonstrated that postpubertal environmental enrichment (EE) rescues and promotes resiliency against MIA in male rats. Importantly, EE protocols have demonstrated clinical relevancy in human rehabilitation settings. Applying some of the elements of these EE protocols (e.g. social, physical, cognitive stimulation) to animal models of health and disease allows for the exploration of the mechanisms that underlie their success. Here, using a MIA model, we further investigate the rehabilitative potential of complex environments with a focus on female animals. Additionally, we expand upon some of our previous work by exploring genetic markers of synaptic plasticity and stress throughout several brain regions of both sexes. In the current study, standard housed female Sprague-Dawley rats were challenged with either the inflammatory endotoxin lipopolysaccharide (LPS; 100 μg/kg) or saline (equivolume) on gestational day 15. On postnatal day 50, male and female offspring were randomized into one of three conditions that differed in terms of cage size, number of cage mates (social stimulation) and enrichment materials. Spatial discrimination ability and social behavior were assessed six weeks later. Similar to our previously published work in males, our results revealed that a single LPS injection during mid gestation disrupted spatial discrimination ability in female rats. Postpubertal EE rescued this disruption. On the endocrine level, EE dampened elevations in plasma corticosterone that followed MIA, which may mediate EE's rehabilitative effects in female offspring. Within the prefrontal cortex, hippocampus, amygdala, and hypothalamus, MIA and EE altered the mRNA expression of several genes associated with resiliency and synaptic plasticity in both sexes. Overall, our findings provide further evidence that EE may serve as a therapeutic intervention for MIA-induced behavioral and cognitive deficits. Moreover, we identify some sexually dimorphic molecular mechanisms that may underlie these impairments and their rescue.
Keywords: Amygdala; Corticosterone; Environmental enrichment; Experience; Fetal programming; Glucocorticoid receptor; Inflammation; Maternal immune activation; Memory; Sex difference; Social Housing; Synaptic plasticity.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Figures





References
-
- Bakos J., Hlavacova N., Rajman M., Ondicova K., Koros C., Kitraki E., Steinbusch H., Jezova D. Enriched environment influences hormonal status and hippocampal brain derived neurotrophic factor in a sex dependent manner. Neuroscience. 2009;164(2):788–797. doi: 10.1016/j.neuroscience.2009.08.054. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

