Switching between Local and Global Aromaticity in a Conjugated Macrocycle for High-Performance Organic Sodium-Ion Battery Anodes
- PMID: 32368821
- PMCID: PMC7496320
- DOI: 10.1002/anie.202003386
Switching between Local and Global Aromaticity in a Conjugated Macrocycle for High-Performance Organic Sodium-Ion Battery Anodes
Abstract
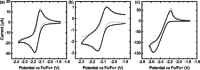
Aromatic organic compounds can be used as electrode materials in rechargeable batteries and are expected to advance the development of both anode and cathode materials for sodium-ion batteries (SIBs). However, most aromatic organic compounds assessed as anode materials in SIBs to date exhibit significant degradation issues under fast-charge/discharge conditions and unsatisfying long-term cycling performance. Now, a molecular design concept is presented for improving the stability of organic compounds for battery electrodes. The molecular design of the investigated compound, [2.2.2.2]paracyclophane-1,9,17,25-tetraene (PCT), can stabilize the neutral state by local aromaticity and the doubly reduced state by global aromaticity, resulting in an anode material with extraordinarily stable cycling performance and outstanding performance under fast-charge/discharge conditions, demonstrating an exciting new path for the development of electrode materials for SIBs and other types of batteries.
Keywords: aromaticity; hydrocarbons; macrocycles; organic batteries; voids.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- None
-
- Shea J. J., Luo C., ACS Appl. Mater. Interfaces 2020, 12, 5361–5380; - PubMed
-
- Heiska J., Nisula M., Karppinen M., J. Mater. Chem. A 2019, 7, 18735–18758.
-
- Larcher D., Tarascon J. M., Nat. Chem. 2015, 7, 19–29. - PubMed
-
- Vaalma C., Buchholz D., Weil M., Passerini S., Nat. Rev. Mater. 2018, 3, 18013.
Grants and funding
LinkOut - more resources
Full Text Sources

