Tubular β-catenin and FoxO3 interactions protect in chronic kidney disease
- PMID: 32369448
- PMCID: PMC7259539
- DOI: 10.1172/jci.insight.135454
Tubular β-catenin and FoxO3 interactions protect in chronic kidney disease
Abstract
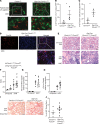
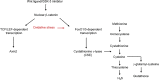
The Wnt/β-catenin signaling pathway plays an important role in renal development and is reexpressed in the injured kidney and other organs. β-Catenin signaling is protective in acute kidney injury (AKI) through actions on the proximal tubule, but the current dogma is that Wnt/β-catenin signaling promotes fibrosis and development of chronic kidney disease (CKD). As the role of proximal tubular β-catenin signaling in CKD remains unclear, we genetically stabilized (i.e., activated) β-catenin specifically in murine proximal tubules. Mice with increased tubular β-catenin signaling were protected in 2 murine models of AKI to CKD progression. Oxidative stress, a common feature of CKD, reduced the conventional T cell factor/lymphoid enhancer factor-dependent β-catenin signaling and augmented FoxO3-dependent activity in proximal tubule cells in vitro and in vivo. The protective effect of proximal tubular β-catenin in renal injury required the presence of FoxO3 in vivo. Furthermore, we identified cystathionine γ-lyase as a potentially novel transcriptional target of β-catenin/FoxO3 interactions in the proximal tubule. Thus, our studies overturned the conventional dogma about β-catenin signaling and CKD by showing a protective effect of proximal tubule β-catenin in CKD and identified a potentially new transcriptional target of β-catenin/FoxO3 signaling that has therapeutic potential for CKD.
Keywords: Cell Biology; Cell stress; Fibrosis; Nephrology.
Conflict of interest statement
Figures






References
-
- United States Renal Data System. 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, Maryland, USA: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

