Grifolin, neogrifolin and confluentin from the terricolous polypore Albatrellus flettii suppress KRAS expression in human colon cancer cells
- PMID: 32369483
- PMCID: PMC7199964
- DOI: 10.1371/journal.pone.0231948
Grifolin, neogrifolin and confluentin from the terricolous polypore Albatrellus flettii suppress KRAS expression in human colon cancer cells
Abstract
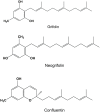
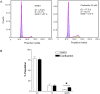
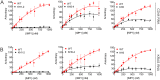
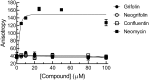
In our search for bioactive mushrooms native to British Columbia, we determined that the ethanol extracts from fruiting bodies of the terrestrial polypore Albatrellus flettii had potent anti-cell viability activity. Using bioassay-guided fractionation, mass spectrometry and nuclear magnetic resonance, we successfully isolated three known compounds (grifolin, neogrifolin and confluentin). These compounds represent the major anti-cell viability components from the ethanol extracts of A. flettii. We also identified a novel biological activity for these compounds, specifically in down-regulating KRAS expression in two human colon cancer cell lines. Relatively little is known about the anti-cell viability activity and mechanism of action of confluentin. For the first time, we show the ability of confluentin to induce apoptosis and arrest the cell cycle at the G2/M phase in SW480 human colon cancer cells. The oncogenic insulin-like growth factor 2 mRNA-binding protein 1 (IMP1) has been previously shown to regulate KRAS mRNA expression in colon cancer cells, possibly through its ability to bind to the KRAS transcript. Using a fluorescence polarization assay, we show that confluentin dose-dependently inhibits the physical interaction between KRAS RNA and full-length IMP1. The inhibition also occurs with truncated IMP1 containing the KH1 to KH4 domain (KH1to4 IMP1), but not with the di-domain KH3 and KH4 (KH3&4 IMP1). In addition, unlike the control antibiotic neomycin, grifolin, neogrifolin and confluentin do not bind to KRAS RNA. These results suggest that confluentin inhibits IMP1-KRAS RNA interaction by binding to the KH1&2 di-domains of IMP1. Since the molecular interaction between IMP1 and its target RNAs is a pre-requisite for the oncogenic function of IMP1, confluentin should be further explored as a potential inhibitor of IMP1 in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Deo G, Khatra J, Buttar S, Li WM, Tackaberry LE, Massicotte H.B, et al. Anti-proliferative, immuno-stimulatory and anti-inflammatory activities of extracts derived from mushrooms collected in Haida Gwaii, British Columbia, Canada. Int J Med Mushrooms. 2019;21: 629–643. 10.1615/IntJMedMushrooms.2019031193 - DOI - PubMed
-
- Ginns J. The taxonomy and distribution of rare or uncommon species of Albatrellus in western North America. Can J Bot. 1997;75: 261–273.
-
- Nukata M, Hashimoto T, Yamamoto I, Iwasaki N, Tanaka M, Asakawa Y. Neogrifolin derivatives possessing anti-oxidative activity from the mushroom Albatrellus ovinus. Phytochem. 2002;59: 731–737. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

