Platelets docking to VWF prevent leaks during leukocyte extravasation by stimulating Tie-2
- PMID: 32369573
- PMCID: PMC7413753
- DOI: 10.1182/blood.2019003442
Platelets docking to VWF prevent leaks during leukocyte extravasation by stimulating Tie-2
Abstract
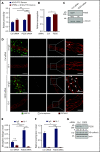
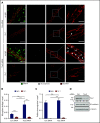
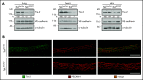
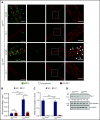
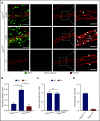
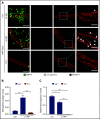
Neutrophil extravasation requires opening of the endothelial barrier but does not necessarily cause plasma leakage. Leaks are prevented by contractile actin filaments surrounding the diapedesis pore, keeping this opening tightly closed around the transmigrating neutrophils. We have identified the receptor system that is responsible for this. We show that silencing, or gene inactivation, of endothelial Tie-2 results in leak formation in postcapillary venules of the inflamed cremaster muscle at sites of neutrophil extravasation, as visualized by fluorescent microspheres. Leakage was dependent on neutrophil extravasation, because it was absent upon neutrophil depletion. We identified the Cdc42 GTPase exchange factor FGD5 as a downstream target of Tie-2 that is essential for leakage prevention during neutrophil extravasation. Looking for the Tie-2 agonist and its source, we found that platelet-derived angiopoietin-1 (Angpt1) was required to prevent neutrophil-induced leaks. Intriguingly, blocking von Willebrand factor (VWF) resulted in vascular leaks during transmigration, indicating that platelets interacting with endothelial VWF activate Tie-2 by secreting Angpt1, thereby preventing diapedesis-induced leakiness.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Comment in
-
Why vessels do not leak when leukocytes migrate out.Blood. 2020 Jul 30;136(5):531-532. doi: 10.1182/blood.2020006568. Blood. 2020. PMID: 32730582 No abstract available.
References
-
- Carden DL, Smith JK, Korthuis RJ. Neutrophil-mediated microvascular dysfunction in postischemic canine skeletal muscle. Role of granulocyte adherence. Circ Res. 1990;66(5):1436-1444. - PubMed
-
- Kubes P, Suzuki M, Granger DN. Modulation of PAF-induced leukocyte adherence and increased microvascular permeability. Am J Physiol. 1990;259(5 Pt 1):G859-G864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

