The selective glucocorticoid receptor antagonist CORT125281 has tissue-specific activity
- PMID: 32369774
- PMCID: PMC7274539
- DOI: 10.1530/JOE-19-0486
The selective glucocorticoid receptor antagonist CORT125281 has tissue-specific activity
Abstract
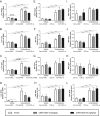
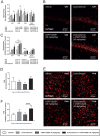
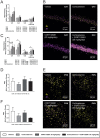
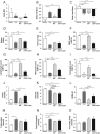
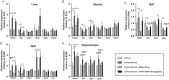
Glucocorticoids mediate numerous essential processes in the human body via binding to the glucocorticoid receptor (GR). Excessive GR signaling can cause disease, and GR antagonists can be used to treat many symptoms of glucocorticoid-induced pathology. The purpose of this study was to characterize the tissue-specific properties of the selective GR antagonist CORT125281. We evaluated the antagonistic effects of CORT125281 upon acute and subchronic corticosterone exposure in mice. In the acute corticosterone setting, hypothalamus-pituitary-adrenal-axis activity was investigated by measurement of basal- and stress-induced corticosterone levels, adrenocorticotropic hormone levels and pituitary proopiomelanocortin expression. GR signaling was evaluated by RT-PCR analysis of GR-responsive transcripts in liver, muscle, brown adipose tissue (BAT), white adipose tissue (WAT) and hippocampus. Pretreatment with a high dose of CORT125281 antagonized GR activity in a tissue-dependent manner. We observed complete inhibition of GR-induced target gene expression in the liver, partial blockade in muscle and BAT and no antagonism in WAT and hippocampus. Tissue distribution only partially explained the lack of effective antagonism. CORT125281 treatment did not disinhibit the hypothalamus-pituitary-adrenal neuroendocrine axis. In the subchronic corticosterone setting, CORT125281 partially prevented corticosterone-induced hyperinsulinemia, but not hyperlipidemia and immune suppression. In conclusion, CORT125281 antagonizes GR transcriptional activity in a tissue-dependent manner and improves corticosterone-induced hyperinsulinemia. Tailored dosing of CORT125281 may allow tissue-specific inhibition of GR transcriptional activity.
Keywords: GR antagonist; HPA-axis; RU486; coregulators; corticosterone; glucocorticoid receptor; mifepristone; tissue-specificity.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

