Virulence Characteristics of mecA-Positive Multidrug-Resistant Clinical Coagulase-Negative Staphylococci
- PMID: 32369929
- PMCID: PMC7284987
- DOI: 10.3390/microorganisms8050659
Virulence Characteristics of mecA-Positive Multidrug-Resistant Clinical Coagulase-Negative Staphylococci
Abstract
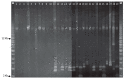
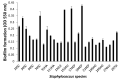
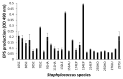
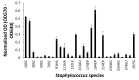
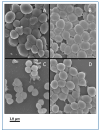
Coagulase-negative staphylococci (CoNS) are an important group of opportunistic pathogenic microorganisms that cause infections in hospital settings and are generally resistant to many antimicrobial agents. We report on phenotypic and genotypic virulence characteristics of a select group of clinical, mecA-positive (encoding penicillin-binding protein 2a) CoNS isolates. All CoNS were resistant to two or more antimicrobials with S. epidermidis strain 214EP, showing resistance to fifteen of the sixteen antimicrobial agents tested. Aminoglycoside-resistance genes were the ones most commonly detected. The presence of megaplasmids containing both horizontal gene transfer and antimicrobial resistance genetic determinants indicates that CoNS may disseminate antibiotic resistance to other bacteria. Staphylococcus sciuri species produced six virulence enzymes, including a DNase, gelatinase, lipase, phosphatase, and protease that are suspected to degrade tissues into nutrients for bacterial growth and contribute to the pathogenicity of CoNS. The PCR assay for the detection of biofilm-associated genes found the eno (encoding laminin-binding protein) gene in all isolates. Measurement of their biofilm-forming ability and Spearman's rank correlation coefficient analyses revealed that the results of crystal violet (CV) and extracellular polymeric substances (EPS) assays were significantly correlated (ρ = 0.9153, P = 3.612e-12). The presence of virulence factors, biofilm-formation capability, extracellular enzymes, multidrug resistance, and gene transfer markers in mecA-positive CoNS clinical strains used in this study makes them powerful opportunistic pathogens. The study also warrants a careful evaluation of nosocomial infections caused by CoNS and may be useful in studying the mechanism of virulence and factors associated with their pathogenicity in vivo and developing effective strategies for mitigation.
Keywords: coagulase-negative staphylococci; multidrug-resistant; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

