Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models
- PMID: 32369940
- PMCID: PMC7247356
- DOI: 10.3390/ijms21093205
Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models
Abstract
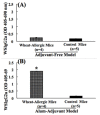
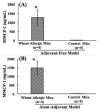
Wheat protein is considered a major type of food allergen in many countries including the USA. The mechanisms of allergenicity of wheat proteins are not well understood at present. Both adjuvant-based and adjuvant-free mouse models are reported for this food allergy. However, it is unclear whether the mechanisms underlying wheat allergenicity in these two types of models are similar or different. Therefore, we compared the molecular mechanisms in a novel adjuvant-free (AF) model vs. a conventional alum-adjuvant (AA) model of wheat allergy using salt-soluble wheat protein (SSWP). In the AF model, Balb/cJ mice were sensitized with SSWP via skin exposure. In the AA model, mice were sensitized by an intraperitoneal injection of SSWP with alum. In both models, allergic reactions were elicited using an identical protocol. Robust IgE as well as mucosal mast cell protein-1 responses were elicited similarly in both models. However, an analysis of the spleen immune markers identified strikingly different molecular activation patterns in these two models. Furthermore, a number of immune markers associated with intrinsic allergenicity were also identified in both models. Since the AF model uses skin exposure without an adjuvant, the mechanisms in the AF model may more closely simulate the human wheat allergenicity mechanisms from skin exposure in occupational settings such as in the baking industry.
Keywords: adjuvant; food allergy; food safety; immune markers; intrinsic allergenicity; mouse model; risk assessment; skin sensitization; wheat allergy; wheat protein allergenicity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Biagini R.E., MacKenzie B.A., Sammons D.L., Smith J.P., Striley C.A., Robertson S.K., Snawder J.E. Evaluation of the prevalence of antiwheat-, anti-flour dust, and anti-alpha-amylase specific IgE antibodies in US blood donors. Ann. Allergy Asthma Immunol. 2004;92:649–653. doi: 10.1016/S1081-1206(10)61431-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

