Synthetic Hydroxyapatite Inhibits Bisphosphonate Toxicity to the Oral Mucosa In Vitro
- PMID: 32369961
- PMCID: PMC7254283
- DOI: 10.3390/ma13092086
Synthetic Hydroxyapatite Inhibits Bisphosphonate Toxicity to the Oral Mucosa In Vitro
Abstract
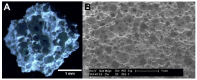
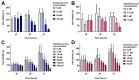
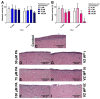
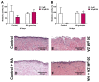
Medication-related osteonecrosis of the jaw (MRONJ) is a side effect of bisphosphonate therapy, characterised by exposed necrotic bone. The soft tissues of the oral mucosa no longer provide a protective barrier and MRONJ patients experience pain, infections and difficulties eating. We hypothesised that hydroxyapatite (Ca5(PO4)3(OH)) could reduce bisphosphonate concentrations and protect the oral mucosa by exploiting bisphosphonate's calcium binding affinity. The effect of zoledronic acid (ZA) and pamidronic acid (PA) on the metabolism of oral fibroblasts, oral keratinocytes and three-dimensional oral mucosa models was investigated and then repeated in the presence of hydroxyapatite granules. Without hydroxyapatite, ZA and PA significantly reduced the metabolic activity of oral cells in a dose-dependent manner. Both drugs reduced epithelial thickness and 30 µM ZA resulted in loss of the epithelium. Hydroxyapatite granules had a protective effect on oral cells, with metabolic activity retained. Oral mucosa models retained their multi-layered epithelium when treated with ZA in the presence of hydroxyapatite granules and metabolic activity was comparable to controls. These results demonstrate hydroxyapatite granules protected oral soft tissues from damage caused by bisphosphonate exposure. Porous hydroxyapatite granules are currently used for socket preservation and this data suggests their potential to prevent MRONJ in at-risk patients.
Keywords: BRONJ; MRONJ; fibroblasts; keratinocytes; osteonecrosis; osteonecrosis of the jaw; pamidronate; pamidronic acid; zoledronate; zoledronic acid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Inflammation Can Be a High-Risk Factor for Mucosal Nonunion of MRONJ by Regulating SIRT1 Signaling When Treated with an Oncologic Dose of Zoledronate.Drug Des Devel Ther. 2024 Jul 4;18:2793-2812. doi: 10.2147/DDDT.S456811. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38979400 Free PMC article.
-
A Review Into the Effects of Pamidronic Acid and Zoledronic Acid on the Oral Mucosa in Medication-Related Osteonecrosis of the Jaw.Front Oral Health. 2022 Feb 9;2:822411. doi: 10.3389/froh.2021.822411. eCollection 2021. Front Oral Health. 2022. PMID: 35224540 Free PMC article. Review.
-
In vitro Effect of Geranylgeraniol (GGOH) on Bisphosphonate-Induced Cytotoxicity of Oral Mucosa Cells.Front Oral Health. 2022 Jun 20;3:892615. doi: 10.3389/froh.2022.892615. eCollection 2022. Front Oral Health. 2022. PMID: 35795156 Free PMC article.
-
Rescue bisphosphonate treatment of alveolar bone improves extraction socket healing and reduces osteonecrosis in zoledronate-treated mice.Bone. 2019 Jun;123:115-128. doi: 10.1016/j.bone.2019.03.027. Epub 2019 Mar 26. Bone. 2019. PMID: 30926440 Free PMC article.
-
Medication-related osteonecrosis of the jaws (MRONJ) in cancer patients treated with denosumab VS. zoledronic acid: A systematic review and meta-analysis.Med Oral Patol Oral Cir Bucal. 2020 May 1;25(3):e326-e336. doi: 10.4317/medoral.23324. Med Oral Patol Oral Cir Bucal. 2020. PMID: 32271321 Free PMC article.
Cited by
-
Inflammation Can Be a High-Risk Factor for Mucosal Nonunion of MRONJ by Regulating SIRT1 Signaling When Treated with an Oncologic Dose of Zoledronate.Drug Des Devel Ther. 2024 Jul 4;18:2793-2812. doi: 10.2147/DDDT.S456811. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38979400 Free PMC article.
-
Zoledronic acid affects the process of Porphyromonas gingivalis infecting oral mucosal epithelial barrier: An in-vivo and in-vitro study.Front Cell Infect Microbiol. 2023 Mar 28;13:1104826. doi: 10.3389/fcimb.2023.1104826. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37056703 Free PMC article.
-
A Review Into the Effects of Pamidronic Acid and Zoledronic Acid on the Oral Mucosa in Medication-Related Osteonecrosis of the Jaw.Front Oral Health. 2022 Feb 9;2:822411. doi: 10.3389/froh.2021.822411. eCollection 2021. Front Oral Health. 2022. PMID: 35224540 Free PMC article. Review.
-
An Overview of Physical, Microbiological and Immune Barriers of Oral Mucosa.Int J Mol Sci. 2021 Jul 22;22(15):7821. doi: 10.3390/ijms22157821. Int J Mol Sci. 2021. PMID: 34360589 Free PMC article. Review.
-
In Vitro Cytotoxicity of Antiresorptive and Antiangiogenic Compounds on Oral Tissues Contributing to MRONJ: Systematic Review.Biomolecules. 2023 Jun 10;13(6):973. doi: 10.3390/biom13060973. Biomolecules. 2023. PMID: 37371553 Free PMC article.
References
-
- Dimopoulos M.A., Kastritis E., Anagnostopoulos A., Melakopoulos I., Gika D., Moulopoulos L.A., Bamia C., Terpos E., Tsionos K., Bamias A. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: Evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91:968–971. - PubMed
-
- Ruggiero S.L., Dodson T.B., Fantasia J., Goodday R., Aghaloo T., Mehrotra B., O’Ryan F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. - DOI - PubMed
-
- Khosla S., Burr D., Cauley J., Dempster D.W., Ebeling P.R., Felsenberg D., Gagel R.F., Gilsanz V., Guise T., Koka S., et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

