Synthetic Biology Approaches to Engineer Saccharomyces cerevisiae towards the Industrial Production of Valuable Polyphenolic Compounds
- PMID: 32370107
- PMCID: PMC7281501
- DOI: 10.3390/life10050056
Synthetic Biology Approaches to Engineer Saccharomyces cerevisiae towards the Industrial Production of Valuable Polyphenolic Compounds
Abstract
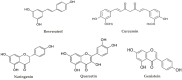
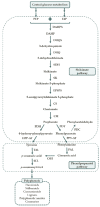
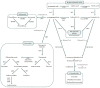
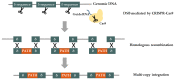
Polyphenols are plant secondary metabolites with diverse biological and potential therapeutic activities such as antioxidant, anti-inflammatory and anticancer, among others. However, their extraction from the native plants is not enough to satisfy the increasing demand for this type of compounds. The development of microbial cell factories to effectively produce polyphenols may represent the most attractive solution to overcome this limitation and produce high amounts of these bioactive molecules. With the advances in the synthetic biology field, the development of efficient microbial cell factories has become easier, largely due to the development of the molecular biology techniques and by the identification of novel isoenzymes in plants or simpler organisms to construct the heterologous pathways. Furthermore, efforts have been made to make the process more profitable through improvements in the host chassis. In this review, advances in the production of polyphenols by genetically engineered Saccharomyces cerevisiae as well as by synthetic biology and metabolic engineering approaches to improve the production of these compounds at industrial settings are discussed.
Keywords: Saccharomyces cerevisiae; heterologous production; metabolic engineering; polyphenols biosynthesis; synthetic biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pagare S., Bhatia M., Tripathi N., Pagare S., Bansal Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2016;9:293–304.
-
- Galanakis C.M. Polyphenols: Properties, Recovery, and Applications. Woodhead Publishing; Sawston, UK: Cambrigde, UK: 2018.
-
- Global Polyphenols Market Will Register a CAGR of Around 9.0% by 2027. [(accessed on 21 April 2020)]; Available online: https://proficientmarket.com/press-release/1225/global-polyphenols-market.
-
- Global Flavonoids Market Will Reach USD 1047.63 Million in 2021. [(accessed on 21 April 2020)]; Available online: https://www.zionmarketresearch.com/news/global-flavonoids-market.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

