Expanding the Spectrum of Adenoviral Vectors for Cancer Therapy
- PMID: 32370135
- PMCID: PMC7281331
- DOI: 10.3390/cancers12051139
Expanding the Spectrum of Adenoviral Vectors for Cancer Therapy
Abstract
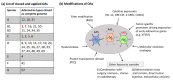
Adenoviral vectors (AdVs) have attracted much attention in the fields of vaccine development and treatment for diseases such as genetic disorders and cancer. In this review, we discuss the utility of AdVs in cancer therapies. In recent years, AdVs were modified as oncolytic AdVs (OAs) that possess the characteristics of cancer cell-specific replication and killing. Different carriers such as diverse cells and extracellular vesicles are being explored for delivering OAs into cancer sites after systemic administration. In addition, there are also various strategies to improve cancer-specific replication of OAs, mainly through modifying the early region 1 (E1) of the virus genome. It has been documented that oncolytic viruses (OVs) function through stimulating the immune system, resulting in the inhibition of cancer progression and, in combination with classical immune modulators, the anti-cancer effect of OAs can be even further enforced. To enhance the cancer treatment efficacy, OAs are also combined with other standard treatments, including surgery, chemotherapy and radiotherapy. Adenovirus type 5 (Ad5) has mainly been explored to develop vectors for cancer treatment with different modulations. Only a limited number of the more than 100 identified AdV types were converted into OAs and, therefore, the construction of an adenovirus library for the screening of potential novel OA candidates is essential. Here, we provide a state-of-the-art overview of currently performed and completed clinic trials with OAs and an adenovirus library, providing novel possibilities for developing innovative adenoviral vectors for cancer treatment.
Keywords: adenoviral vectors; adenovirus; adenovirus library; cancer; chemotherapy; delivery; immunotherapy; oncolytic adenovirus; radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- HAdV Working Group. [(accessed on 2 May 2020)]; Available online: http://hadvwg.gmu.edu/
-
- Hage E., Gerd Liebert U., Bergs S., Ganzenmueller T., Heim A. Human mastadenovirus type 70: A novel, multiple recombinant species D mastadenovirus isolated from diarrhoeal faeces of a haematopoietic stem cell transplantation recipient. J. Gen. Virol. 2015;96:2734–2742. doi: 10.1099/vir.0.000196. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

