A Replication-Defective Influenza Virus Vaccine Confers Complete Protection against H7N9 Viral Infection in Mice
- PMID: 32370136
- PMCID: PMC7349114
- DOI: 10.3390/vaccines8020207
A Replication-Defective Influenza Virus Vaccine Confers Complete Protection against H7N9 Viral Infection in Mice
Abstract
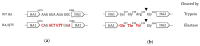
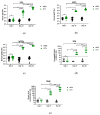
Avian influenza H7N9 viruses continue to pose a great threat to public health, which is evident by their high case-fatality rates. Although H7N9 was first isolated in humans in China in 2013, to date, there is no commercial vaccine available against this particular strain. Our previous studies developed a replication-defective influenza virus through mutation of the hemagglutinin (HA) cleavage site from a trypsin-sensitive to an elastase-sensitive motif. In this study, we report the development of a reassortant mutant influenza virus derived from the human isolate A/British Columbia/01/2015 (H7N9) [BC15 (H7N9)], which is the QVT virus. The HA gene of this virus possesses three mutations at the cleavage site, Lys-Gly-Arg were mutated to Gln-Thr-Val at amino acid (aa) positions 337, 338, and 339, respectively. We report this virus to rely on elastase in vitro, possess unaltered replication abilities when elastase was provided compared to the wild type virus in vitro, and to be non-virulent and replication-defective in mice. In addition, we report this virus to induce significant levels of antibodies and IFN-γ and IL-5 secreting cells, and to protect mice against a lethal challenge of the BC15 (H7N9) virus. This protection is demonstrated through the lack of body weight loss, 100% survival rate, and the prevention of BC15 (H7N9) viral replication as well as the reduction of proinflammatory cytokines induced in the mouse lung associated with the influenza disease. Therefore, these results provide strong evidence for the use of this reassortant mutant H7N9 virus as a replication-defective virus vaccine candidate against H7N9 viruses.
Keywords: elastase dependent virus; influenza A virus H7N9; replication-defective virus vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- FAO H7N9 Situation Update. [(accessed on 15 March 2020)]; Available online: http://www.fao.org/ag/againfo/programmes/en/empres/h7n9/situation_update....
-
- Yang Y., Wong G., Yang L., Tan S., Li J., Bai B., Xu Z., Li H., Xu W., Zhao X., et al. Comparison between human infections caused by highly and low pathogenic H7N9 avian influenza viruses in Wave Five: Clinical and virological findings. J. Infect. 2019;78:241–248. doi: 10.1016/j.jinf.2019.01.005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

