Eumelanin Precursor 2-Carboxy-5,6-Dihydroxyindole (DHICA) as Doping Factor in Ternary (PEDOT:PSS/Eumelanin) Thin Films for Conductivity Enhancement
- PMID: 32370189
- PMCID: PMC7254328
- DOI: 10.3390/ma13092108
Eumelanin Precursor 2-Carboxy-5,6-Dihydroxyindole (DHICA) as Doping Factor in Ternary (PEDOT:PSS/Eumelanin) Thin Films for Conductivity Enhancement
Abstract
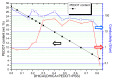
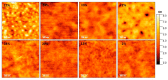
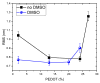
The integration of the pristine not-doped commercial poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) PH1000 with eumelanin, the brown to black kind of melanin pigment, was achieved by dissolving the melanogenic precursors 2-carboxy-5,6-dihydroxyindole (DHICA) in the PH1000 suspension. Solid state oxidative polymerization of the catecholic indole allowed obtaining the ternary blend PEDOT:PSS/eumelanin. The introduction of DHICA into PH1000 produced a noticeable increase in the conductivity of PEDOT thin films akin to that produced by dimethyl sulfoxide (DMSO) treatment, opening up novel strategies for the simultaneous integration of eumelanin polymer and conductivity enhancement of PEDOT containing coatings, as well as the long term goal of replacing PSS by DHICA eumelanin for PEDOT pairing.
Keywords: PEDOT:PSS; bioinspired materials; conducting polymer; eumelanin; melanin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Lee H.B., Jin W.Y., Ovhal M.M., Kumar N., Kang J.W. Flexible transparent conducting electrodes based on metal meshes for organic optoelectronic device applications: A review. J. Mater. Chem. C. 2019;7:1087–1110. doi: 10.1039/C8TC04423F. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

