ROCK (RhoA/Rho Kinase) in Cardiovascular-Renal Pathophysiology: A Review of New Advancements
- PMID: 32370294
- PMCID: PMC7290501
- DOI: 10.3390/jcm9051328
ROCK (RhoA/Rho Kinase) in Cardiovascular-Renal Pathophysiology: A Review of New Advancements
Abstract
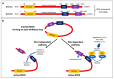
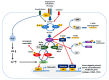
Rho-associated, coiled-coil containing kinases (ROCK) were originally identified as effectors of the RhoA small GTPase and found to belong to the AGC family of serine/threonine kinases. They were shown to be downstream effectors of RhoA and RhoC activation. They signal via phosphorylation of proteins such as MYPT-1, thereby regulating many key cellular functions including proliferation, motility and viability and the RhoA/ROCK signaling has been shown to be deeply involved in arterial hypertension, cardiovascular-renal remodeling, hypertensive nephropathy and posttransplant hypertension. Given the deep involvement of ROCK in cardiovascular-renal pathophysiology and the interaction of ROCK signaling with other signaling pathways, the reports of trials on the clinical beneficial effects of ROCK's pharmacologic targeting are growing. In this current review, we provide a brief survey of the current understanding of ROCK-signaling pathways, also integrating with the more novel data that overall support a relevant role of ROCK for the cardiovascular-renal physiology and pathophysiology.
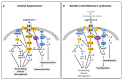
Keywords: Bartter’s syndrome; Gitelman’s syndrome; ROCK; Rho; Rho kinase; cardiovascular remodeling; hypertension; hypertensive nephropathy; kidney remodeling; posttransplant hypertension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

