Multi-tiered screening and diagnosis strategy for COVID-19: a model for sustainable testing capacity in response to pandemic
- PMID: 32370561
- PMCID: PMC7877955
- DOI: 10.1080/07853890.2020.1763449
Multi-tiered screening and diagnosis strategy for COVID-19: a model for sustainable testing capacity in response to pandemic
Abstract
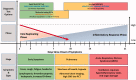
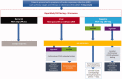
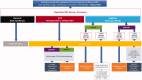
Coronavirus disease 2019 (COVID-19), caused by novel enveloped single stranded RNA coronavirus (SARS-CoV-2), is responsible for an ongoing global pandemic. While other countries deployed widespread testing as an early mitigation strategy, the U.S. experienced delays in development and deployment of organism identification assays. As such, there is uncertainty surrounding disease burden and community spread, severely hampering containment efforts. COVID-19 illuminates the need for a tiered diagnostic approach to rapidly identify clinically significant infections and reduce disease spread. Without the ability to efficiently screen patients, hospitals are overwhelmed, potentially delaying treatment for other emergencies. A multi-tiered, diagnostic strategy incorporating a rapid host immune response assay as a screening test, molecular confirmatory testing and rapid IgM/IgG testing to assess benefit from quarantine/further testing and provide information on population exposure/herd immunity would efficiently evaluate potential COVID-19 patients. Triaging patients within minutes with a fingerstick rather than hours/days after an invasive swab is critical to pandemic response as reliance on the existing strategy is limited by assay accuracy, time to results, and testing capacity. Early screening and triage is achievable from the outset of a pandemic with point-of-care host immune response testing which will improve response time to clinical and public health actions.Key messagesDelayed testing deployment has led to uncertainty surrounding overall disease burden and community spread, severely hampering public health containment and healthcare system preparation efforts.A multi-tiered testing strategy incorporating rapid, host immune point-of-care tests can be used now and for future pandemic planning by effectively identifying patients at risk of disease thereby facilitating quarantine earlier in the progression of the outbreak during the weeks and months it can take for pathogen specific confirmatory tests to be developed, validated and manufactured in sufficient quantities.The ability to triage patients at the point of care and support the guidance of medical and therapeutic decisions, for viral isolation or confirmatory testing or for appropriate treatment of COVID-19 and/or bacterial infections, is a critical component to our national pandemic response and there is an urgent need to implement the proposed strategy to combat the current outbreak.
Keywords: C-reactive protein (CRP); COVID-19; Coronavirus; SARS-CoV-2; molecular diagnostics; myxovirus resistance protein A (MxA); point-of-care test; rapid diagnostic tests; serology.
Conflict of interest statement
Financial support was not received. MSP reports grant money to the University of Wisconsin Madison to conduct research conceived and sponsored by Roche Molecular Systems, Inc. and Rapid Pathogen Screening, Inc. (a wholly owned subsidiary of Lumos Diagnostics, Inc.) PCH is a site principle investigator for a Rapid Pathogen Screening, Inc. sponsored clinical trial. RS is the Chief Medical Officer of Lumos Diagnostics. TPO, AS report no conflicts of interest.
Figures
References
-
- Harris AM, Hicks LA, Qaseem A; for the High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention . Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425–434. - PubMed
-
- Pfuntner A, Wier L, Stocks C. Most frequent conditions in US hospitals, 2011: statistical brief #162. 2006. - PubMed
-
- Centers for Disease Control and Prevention (CDC) . Key facts about influenza (Flu). [accessed 2020 Mar 15]. Available from: https://www.cdc.gov/flu/about/keyfacts.htm
-
- Ksiazek TG, Erdman D, Goldsmith CS, et al. . A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
