Real-world treatment patterns, healthcare resource use and clinical outcomes of patients receiving second line therapy for advanced or metastatic gastric cancer
- PMID: 32370803
- PMCID: PMC7201990
- DOI: 10.1186/s12876-020-01232-z
Real-world treatment patterns, healthcare resource use and clinical outcomes of patients receiving second line therapy for advanced or metastatic gastric cancer
Abstract
Background: Second-line (2 L) chemotherapies for advanced or metastatic gastric cancer have shown improved survival but there is no commonly accepted standard of care. This study examines real-world patient characteristics, treatment patterns, healthcare resource use (HCRU) and clinical outcomes in this setting.
Methods: Retrospective chart reviews were performed at participating institutions from Australia, Canada, Italy and UK for adult patients receiving 2 L treatment for advanced/metastatic disease from January 2013 to July 2015. Data were collected for 12 months or until death.
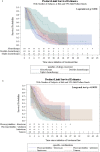
Results: Two hundred eighty patients were included, mean age was 60.9 years and 68.9% were male. Half (51.8%) received monotherapy in 2 L, of whom 69.0% received taxanes. Irinotecan monotherapy was common in Australia (30.0% of monotherapy patients) and Canada (43.8%), but infrequent in Italy and UK. Doublet chemotherapy was used in 36.4% of 2 L patients, most commonly fluoropyrimidine + irinotecan. Use of targeted therapies (trastuzumab, ramucirumab) was infrequent except in Italy. Estimated median real-world progression-free survival (rwPFS) and real-world overall survival (rwOS) from the time of 2 L treatment initiation was 3.09 (95% CI: 2.76-3.68) and 6.54 (5.29-7.76) months, respectively, and estimated 12-month rwPFS and rwOS rate was 8 and 26%, respectively. Only a minority (26.8%) of patients were hospitalized during the follow-up period, with the lowest hospitalization in Italy (16.7%). Laboratory and imaging tests were performed for 93.2 and 70.4%, respectively.
Conclusions: About half of patients received monotherapy as 2 L chemotherapy for advanced/metastatic gastric cancer and a third received doublets. Real-world clinical outcomes for 2 L treatment are poor and HCRU is considerable.
Keywords: Chemotherapy; Gastric cancer; Health care utilization; Outcomes.
Conflict of interest statement
DG reports that IQVIA, employer of DG, received consulting fees from Merck & Co, Inc. during the conduct of the study. MA reports being a full time employee and stockholder of Merck & Co. Inc. during the conduct of the study. SK reports being a full time employee of Merck & Co. Inc. during the conduct of the study. IC reports personal fees from Merck Sharp & Dohme Corp. during the conduct of the study; grants, personal fees and advisory board fees from Eli-Lilly, personal fees from Bristol Meyer Squibb, personal fees from Bayer, grants and personal fees from Merck Serono, personal fees from Roche, personal fees from Astra-Zeneca, grants from Janssen-Cilag, grants from Sanofi, fees from Five Prime Therapeutics, personal fees from Oncologie International, outside the submitted work. JZ reports grants and personal fees from Bayer, personal fees and travel support from Merck & Co. Inc., personal fees and travel support from Merck Sharp & Dohme Corp, outside the submitted work. NL reports that IQVIA, employer of NL, received consulting fees from Merck & Co, Inc. during the conduct of the study. AF reports personal fees from Servier during the conduct of the study; grants and personal fees from Roche, grants and personal fees from Bayer, grants and personal fees from Merck & Co. Inc., grants and personal fees from Amgen, personal fees from Servier, grants from Merck Sharp & Dohme Corp, personal fees from Bristol, personal fees from Lilly, outside the submitted work. All remaining authors have no conflicts of interest to declare.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer – a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47(15):2306–2314. doi: 10.1016/j.ejca.2011.06.002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

