Essential role for autophagy protein ATG7 in the maintenance of intestinal stem cell integrity
- PMID: 32371487
- PMCID: PMC7245075
- DOI: 10.1073/pnas.1917174117
Essential role for autophagy protein ATG7 in the maintenance of intestinal stem cell integrity
Abstract
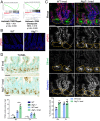
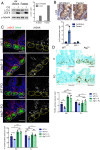
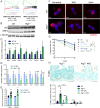
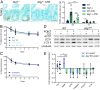
The intestinal epithelium acts as a barrier between the organism and its microenvironment, including the gut microbiota. It is the most rapidly regenerating tissue in the human body thanks to a pool of intestinal stem cells (ISCs) expressing Lgr5 The intestinal epithelium has to cope with continuous stress linked to its digestive and barrier functions. Epithelial repair is crucial to maintain its integrity, and Lgr5-positive intestinal stem cell (Lgr5+ISC) resilience following cytotoxic stresses is central to this repair stage. We show here that autophagy, a pathway allowing the lysosomal degradation of intracellular components, plays a crucial role in the maintenance and genetic integrity of Lgr5+ISC under physiological and stress conditions. Using conditional mice models lacking the autophagy gene Atg7 specifically in all intestinal epithelial cells or in Lgr5+ISC, we show that loss of Atg7 induces the p53-mediated apoptosis of Lgr5+ISC. Mechanistically, this is due to increasing oxidative stress, alterations to interactions with the microbiota, and defective DNA repair. Following irradiation, we show that Lgr5+ISC repair DNA damage more efficiently than their progenitors and that this protection is Atg7 dependent. Accordingly, we found that the stimulation of autophagy on fasting protects Lgr5+ISC against DNA damage and cell death mediated by oxaliplatin and doxorubicin treatments. Finally, p53 deletion prevents the death of Atg7-deficient Lgr5+ISC but promotes genetic instability and tumor formation. Altogether, our findings provide insights into the mechanisms underlying maintenance and integrity of ISC and highlight the key functions of Atg7 and p53.
Keywords: Atg7; DNA repair; autophagy; intestinal stem cells.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Notoginsenoside R1 promotes Lgr5+ stem cell and epithelium renovation in colitis mice via activating Wnt/β-Catenin signaling.Acta Pharmacol Sin. 2024 Jul;45(7):1451-1465. doi: 10.1038/s41401-024-01250-7. Epub 2024 Mar 15. Acta Pharmacol Sin. 2024. PMID: 38491161 Free PMC article.
-
Krüppel-like Factor 5 Regulates Stemness, Lineage Specification, and Regeneration of Intestinal Epithelial Stem Cells.Cell Mol Gastroenterol Hepatol. 2020;9(4):587-609. doi: 10.1016/j.jcmgh.2019.11.009. Epub 2019 Nov 25. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31778829 Free PMC article.
-
1,25-Dihydroxyvitamin D Enhances the Regenerative Function of Lgr5+ Intestinal Stem Cells In Vitro and In Vivo.Cells. 2024 Aug 31;13(17):1465. doi: 10.3390/cells13171465. Cells. 2024. PMID: 39273035 Free PMC article.
-
Plasticity of Intestinal Epithelium: Stem Cell Niches and Regulatory Signals.Int J Mol Sci. 2020 Dec 31;22(1):357. doi: 10.3390/ijms22010357. Int J Mol Sci. 2020. PMID: 33396437 Free PMC article. Review.
-
Reactive Oxygen Species in Modulating Intestinal Stem Cell Dynamics and Function.Stem Cell Rev Rep. 2022 Oct;18(7):2328-2350. doi: 10.1007/s12015-022-10377-1. Epub 2022 Apr 24. Stem Cell Rev Rep. 2022. PMID: 35461466 Review.
Cited by
-
Autophagic state prospectively identifies facultative stem cells in the intestinal epithelium.EMBO Rep. 2022 Nov 7;23(11):e55209. doi: 10.15252/embr.202255209. Epub 2022 Sep 19. EMBO Rep. 2022. PMID: 36120829 Free PMC article.
-
Autophagy in PDGFRα+ mesenchymal cells is essential for intestinal stem cell survival.Proc Natl Acad Sci U S A. 2022 May 24;119(21):e2202016119. doi: 10.1073/pnas.2202016119. Epub 2022 May 10. Proc Natl Acad Sci U S A. 2022. PMID: 35537042 Free PMC article.
-
The emerging roles of autophagy in intestinal epithelial cells and its links to inflammatory bowel disease.Biochem Soc Trans. 2023 Apr 26;51(2):811-826. doi: 10.1042/BST20221300. Biochem Soc Trans. 2023. PMID: 37052218 Free PMC article.
-
Metabolic regulation of the hallmarks of stem cell biology.Cell Stem Cell. 2024 Feb 1;31(2):161-180. doi: 10.1016/j.stem.2024.01.003. Cell Stem Cell. 2024. PMID: 38306993 Free PMC article. Review.
-
Targeting Autophagy to Treat Cancer: Challenges and Opportunities.Front Pharmacol. 2020 Nov 23;11:590344. doi: 10.3389/fphar.2020.590344. eCollection 2020. Front Pharmacol. 2020. PMID: 33381037 Free PMC article. Review.
References
-
- Barker N., Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19–33 (2014). - PubMed
-
- Metcalfe C., Kljavin N. M., Ybarra R., de Sauvage F. J., Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14, 149–159 (2014). - PubMed
-
- Barker N. et al. ., Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009). - PubMed
-
- Mizushima N., A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 20, 521–527 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

