Direct observation of helicase-topoisomerase coupling within reverse gyrase
- PMID: 32371489
- PMCID: PMC7245102
- DOI: 10.1073/pnas.1921848117
Direct observation of helicase-topoisomerase coupling within reverse gyrase
Abstract
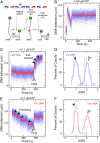
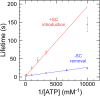
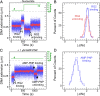
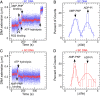
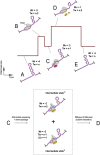
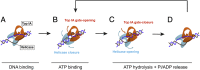
Reverse gyrases (RGs) are the only topoisomerases capable of generating positive supercoils in DNA. Members of the type IA family, they do so by generating a single-strand break in substrate DNA and then manipulating the two single strands to generate positive topology. Here, we use single-molecule experimentation to reveal the obligatory succession of steps that make up the catalytic cycle of RG. In the initial state, RG binds to DNA and unwinds ∼2 turns of the double helix in an ATP-independent fashion. Upon nucleotide binding, RG then rewinds ∼1 turn of DNA. Nucleotide hydrolysis and/or product release leads to an increase of 2 units of DNA writhe and resetting of the enzyme, for a net change of topology of +1 turn per cycle. Final dissociation of RG from DNA results in rewinding of the 2 turns of DNA that were initially disrupted. These results show how tight coupling of the helicase and topoisomerase activities allows for induction of positive supercoiling despite opposing torque.
Keywords: DNA topoisomerase; helicase; magnetic tweezers; single molecule.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Reverse gyrase transiently unwinds double-stranded DNA in an ATP-dependent reaction.J Mol Biol. 2013 Jan 9;425(1):32-40. doi: 10.1016/j.jmb.2012.10.016. Epub 2012 Nov 1. J Mol Biol. 2013. PMID: 23123378
-
Adenosine 5'-O-(3-thio)triphosphate (ATPgammaS) promotes positive supercoiling of DNA by T. maritima reverse gyrase.J Mol Biol. 2007 Aug 3;371(1):197-209. doi: 10.1016/j.jmb.2007.05.031. Epub 2007 May 18. J Mol Biol. 2007. PMID: 17560602
-
The conformational flexibility of the helicase-like domain from Thermotoga maritima reverse gyrase is restricted by the topoisomerase domain.Biochemistry. 2011 Jul 5;50(26):5816-23. doi: 10.1021/bi200236a. Epub 2011 Jun 10. Biochemistry. 2011. PMID: 21627332
-
Helicase-appended topoisomerases: new insight into the mechanism of directional strand transfer.J Biol Chem. 2009 Nov 6;284(45):30737-41. doi: 10.1074/jbc.R109.051268. Epub 2009 Sep 2. J Biol Chem. 2009. PMID: 19726668 Free PMC article. Review.
-
The mechanism of negative DNA supercoiling: a cascade of DNA-induced conformational changes prepares gyrase for strand passage.DNA Repair (Amst). 2014 Apr;16:23-34. doi: 10.1016/j.dnarep.2014.01.011. Epub 2014 Feb 22. DNA Repair (Amst). 2014. PMID: 24674625 Review.
Cited by
-
Variation of Structure and Cellular Functions of Type IA Topoisomerases across the Tree of Life.Cells. 2024 Mar 21;13(6):553. doi: 10.3390/cells13060553. Cells. 2024. PMID: 38534397 Free PMC article. Review.
-
Archaea: A Gold Mine for Topoisomerase Diversity.Front Microbiol. 2021 May 25;12:661411. doi: 10.3389/fmicb.2021.661411. eCollection 2021. Front Microbiol. 2021. PMID: 34113328 Free PMC article. Review.
-
DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis.Bioessays. 2021 Apr;43(4):e2000286. doi: 10.1002/bies.202000286. Epub 2021 Jan 22. Bioessays. 2021. PMID: 33480441 Free PMC article. Review.
-
Nucleosome Dynamics Derived at the Single-Molecule Level Bridges Its Structures and Functions.JACS Au. 2024 Feb 26;4(3):866-876. doi: 10.1021/jacsau.3c00658. eCollection 2024 Mar 25. JACS Au. 2024. PMID: 38559720 Free PMC article. Review.
-
How do neurons live long and healthy? The mechanism of neuronal genome integrity.Front Neurosci. 2025 Mar 19;19:1552790. doi: 10.3389/fnins.2025.1552790. eCollection 2025. Front Neurosci. 2025. PMID: 40177377 Free PMC article. Review.
References
-
- Kikuchi A., Asai K., Reverse gyrase—a topoisomerase which introduces positive superhelical turns into DNA. Nature 309, 677–681 (1984). - PubMed
-
- Nadal M., Mirambeau G., Forterre P., Reiter W. D., Duguet M., Positively supercoiled DNA in a virus-like particle of an archaebacterium. Nature 321, 256–258 (1986).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

