p53 is regulated by aerobic glycolysis in cancer cells by the CtBP family of NADH-dependent transcriptional regulators
- PMID: 32371497
- PMCID: PMC7244340
- DOI: 10.1126/scisignal.aau9529
p53 is regulated by aerobic glycolysis in cancer cells by the CtBP family of NADH-dependent transcriptional regulators
Abstract
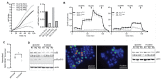
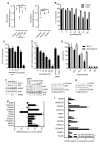
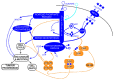
High rates of glycolysis in cancer cells are a well-established characteristic of many human tumors, providing rapidly proliferating cancer cells with metabolites that can be used as precursors for anabolic pathways. Maintenance of high glycolytic rates depends on the lactate dehydrogenase-catalyzed regeneration of NAD+ from GAPDH-generated NADH because an increased NADH:NAD+ ratio inhibits GAPDH. Here, using human breast cancer cell models, we identified a pathway in which changes in the extramitochondrial-free NADH:NAD+ ratio signaled through the CtBP family of NADH-sensitive transcriptional regulators to control the abundance and activity of p53. NADH-free forms of CtBPs cooperated with the p53-binding partner HDM2 to suppress p53 function, and loss of these forms in highly glycolytic cells resulted in p53 accumulation. We propose that this pathway represents a "glycolytic stress response" in which the initiation of a protective p53 response by an increased NADH:NAD+ ratio enables cells to avoid cellular damage caused by mismatches between metabolic supply and demand.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures




References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. - PubMed
-
- Mullarky E, Cantley LC. In: Innovative Medicine: Basic Research and Development. Nakao K, Minato N, Uemoto S, editors. Tokyo: 2015. pp. 3–23. - PubMed
-
- Lehninger AL, Nelson DL, Cox MM. Principles of Biochemistry. ed. 2. Worth; New York: 1992.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

