Physicians' perceptions of the uptake of biosimilars: a systematic review
- PMID: 32371511
- PMCID: PMC7228507
- DOI: 10.1136/bmjopen-2019-034183
Physicians' perceptions of the uptake of biosimilars: a systematic review
Abstract
Objectives: To examine physicians' perceptions of the uptake of biosimilars.
Design: Systematic review.
Data sources: MedLine Ovid and Scopus databases at the end of 2018.
Eligibility criteria: Original scientific studies written in English that addressed physicians' perceptions of the uptake of biosimilars.
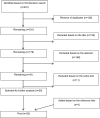
Data extraction and synthesis: The search resulted in altogether 451 studies and 331 after removing duplicates. Two researchers examined these based on the title, abstract and entire text, resulting in 20 studies. The references in these 20 studies were screened and three further studies were included. The data of these 23 studies were extracted. All the publications were quality assessed by two researchers.
Results: Most of the selected studies were conducted in Europe and commonly used short surveys. Physicians' familiarity with biosimilars varied: 49%-76% were familiar with biosimilars while 2%-25% did not know what biosimilars were, the percentages varying from study to study. Their measured knowledge was generally more limited compared with their self-assessed knowledge. Physicians' perceptions of biosimilars also varied: 54%-94% were confident prescribing biosimilars, while 65%-67% had concerns regarding these medicines. Physicians seemed to prefer originator products to biosimilars and prescribed biosimilars mainly for biologic-naive patients. They considered cost savings and the lower price compared with the originator biologic medicine as the main advantages of biosimilars, while their doubts were often related to safety, efficacy and immunogenicity. 64%-95% of physicians had negative perceptions of pharmacist-led substitution of biologic medicines.
Conclusions: Physicians' knowledge of and attitudes towards biosimilars vary. Although physicians had positive attitudes towards biosimilars, prescribing was limited, especially for patients already being treated with biologic medicines. Perceptions of pharmacist-led substitution of biologic medicines were often negative. Education and national recommendations for switching and substitution of biologic medicines are needed to support the uptake of biosimilars.
Keywords: biologic medicine; biosimilar; perception; physician.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Pharmacists' Perspectives of Biosimilars: A Systematic Review.BioDrugs. 2022 Jul;36(4):489-508. doi: 10.1007/s40259-022-00541-x. Epub 2022 Jul 1. BioDrugs. 2022. PMID: 35776294
-
Physicians' and patients' perception of biosimilars and factors affecting biosimilar prescribing in selected Asian countries: a survey study.Expert Opin Biol Ther. 2024 Oct;24(10):1171-1182. doi: 10.1080/14712598.2024.2400523. Epub 2024 Sep 17. Expert Opin Biol Ther. 2024. PMID: 39234698
-
Medical specialists' attitudes to prescribing biosimilars.Pharmacoepidemiol Drug Saf. 2017 May;26(5):570-577. doi: 10.1002/pds.4186. Epub 2017 Feb 24. Pharmacoepidemiol Drug Saf. 2017. PMID: 28233367
-
Real-world familiarity with US biosimilar regulatory guidelines and interchangeability state laws among pharmacists and physicians treating immunological disorders.J Manag Care Spec Pharm. 2025 Jun;31(6):552-564. doi: 10.18553/jmcp.2025.31.6.552. J Manag Care Spec Pharm. 2025. PMID: 40443002 Free PMC article.
-
Factors Affecting Health Care Provider Knowledge and Acceptance of Biosimilar Medicines: A Systematic Review.J Manag Care Spec Pharm. 2019 Jan;25(1):102-112. doi: 10.18553/jmcp.2019.25.1.102. J Manag Care Spec Pharm. 2019. PMID: 30589628 Free PMC article.
Cited by
-
Treatment journey in rheumatoid arthritis with biosimilars: from better access to good disease control through cost savings and prevention of nocebo effects.RMD Open. 2021 Jun;7(2):e001637. doi: 10.1136/rmdopen-2021-001637. RMD Open. 2021. PMID: 34099538 Free PMC article. Review.
-
Regulatory Information and Guidance on Biosimilars and Their Use Across Europe: A Call for Strengthened One Voice Messaging.Front Med (Lausanne). 2022 Mar 9;9:820755. doi: 10.3389/fmed.2022.820755. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35355594 Free PMC article.
-
Pharmacists' Perspectives of Biosimilars: A Systematic Review.BioDrugs. 2022 Jul;36(4):489-508. doi: 10.1007/s40259-022-00541-x. Epub 2022 Jul 1. BioDrugs. 2022. PMID: 35776294
-
Pharmacists' confidence in explaining biosimilars to patients before a nationwide medicine change: A cross-sectional study.Explor Res Clin Soc Pharm. 2022 Oct 25;8:100199. doi: 10.1016/j.rcsop.2022.100199. eCollection 2022 Dec. Explor Res Clin Soc Pharm. 2022. PMID: 36386274 Free PMC article.
-
Retrospective Cohort Study Comparing Infliximab-dyyb and Infliximab in Biologic-Naive Patients With Inflammatory Bowel Disease in the United States.Crohns Colitis 360. 2021 Jul 29;3(3):otab051. doi: 10.1093/crocol/otab051. eCollection 2021 Jul. Crohns Colitis 360. 2021. PMID: 36776661 Free PMC article.
References
-
- European Commission Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the community code relating to medicinal products for human use, 2001.
-
- United States Food and Drug Administration Biosimilar and interchangeable products, 2018. a. Available: www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandAppr... [Accessed 27 Jun 2019].
-
- European Medicines Agency Biosimilar medicines: overview, 2018. a. Available: www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines [Accessed 27 Jun 2019].
-
- QuintilesIMS 2017 IMS Biosimilar Report—The Impact of Biosimilar Competition in Europe, 2017. Available: http://ec.europa.eu/DocsRoom/documents/23102 [Accessed 27 Jun 2019].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources